_________________________________________________________________________________
_________________________________________________________________________________
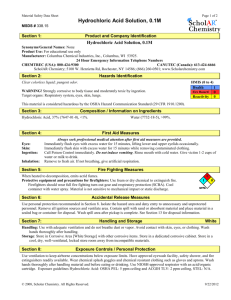
Product Safety Summary Sheet
DuPont™ Hydrochloric Acid
________________________________________________________________________________________
Chemical Identification, Product Identification or Common Name:
CAS number:
EC number:
EC name:
Common name:
7647-01-0
231-791-2
Hydrogen chloride
Muriatic acid
Product Uses and Applications:
Hydrochloric acid (HCl) is primarily manufactured from the reaction of chlorine and hydrogen, both
of which are prepared by the electrolysis of salt solutions, or isolated as a by-product of many
organic or inorganic reactions.
Hydrochloric acid is a strong inorganic acid which is used in a wide variety of industries and
applications. For example, it is:
- produced during the manufacture of various fluorinated gases,
- used in some household cleaning products,
- used in various other industrial applications.
Physical Properties of the Chemical or Product:
Hydrochloric acid is commonly used as an aqueous solution of hydrogen chloride in water, while
hydrogen chloride (HCl) itself is a colorless, non-flammable and corrosive gas at room temperature.
Hydrochloric acid is a highly acidic, corrosive and non-flammable liquid; the typical concentration is
in the range of 30 % HCl by weight (commonly called concentrated hydrochloric acid), but solutions
of up to 38 % (commonly called fuming hydrochloric acid) and lower concentrations of hydrochloric
acid are also available.
DuPont™ Hydrochloric Acid
Page 1 of 4
Revised September 14, 2012
Property
Physical state
Color
Odor
Density at 20 °C
Melting temperature
Boiling temperature
Hydrogen Chloride (HCl) gas
Gas
Colorless
Sharp acidic odor
1.64 g/L (gas is heavier than air)
-112 °C
-85 °C
Hydrochloric acid (30 % HCl)
liquid
Colorless
acidic odor
1.149
approximately -50 °C
approximately 110 °C
Exposure Potential:
Workplace exposure: Hydrochloric acid, when used in industrial settings, is handled in enclosed
systems where there is little to no exposure potential due to its corrosive nature. However, there is
potential for employee exposure during the loading or unloading of transportation vessels or
unintended releases or spills. Exposure to hydrochloric acid in these circumstances could result in
skin or eye contact with the liquid or the inhalation of the vapor phase. These vapors must be
monitored and controlled below the applicable occupational exposure limits(s).
Professional and industrial workers should follow the recommended safety measures contained
within the (Material) Safety Data Sheet ((M)SDS) and on any product packaging. Employees should
be trained in the appropriate work processes and safety equipment to limit exposure to chemical
substances. Occupational use of this substance is considered to be safe provided the recommended
safety measures in the (M)SDS are followed.
Consumer exposure: Consumers can come into contact with the substance through its use as a
component of some household cleaners and in some construction applications where hydrogen
chloride gas can be released. Consumers need to be aware of the corrosive nature of the substance.
Avoid direct contact with skin and eyes. Do not inhale the substance.
Consumer products typically contain only small concentration of hydrochloric acid (1-2 %). This use
has been assessed as safe, provided that the product is used as directed on the label, following all
instructions found on the packaging.
Environmental exposure:
- As a gas: Hydrogen chloride release into the environment does not normally occur since the gas is
used only by industry within closed systems under controlled conditions.
- As a liquid: Hydrochloric acid lowers the pH of any water into which it is released. Industrial
waste-water containing hydrochloric acid is neutralized by the addition of a basic (alkaline)
substance to render it a neutral solution which can be safely disposed. If a large amount of
hydrochloric acid is released or if other acids are present, the pH could be lowered until it is harmful
for aquatic animals and plants (at pH 3 to 5). As this is a generic pH effect and is not a substance
unique effect of hydrochloric acid, it is not classified as toxic to the aquatic environment.
DuPont™ Hydrochloric Acid
Page 2 of 4
Revised September 14, 2012
- As a diluted liquid: Small-scale release into drains from household cleaning products poses
negligible risk to the environment due to the large dilution of the acid and neutralization by the
buffering capacity of the system or natural waters.
Health Information:
Note: The information contained in this section may be useful to someone handling the non
formulated, undiluted substance such as a manufacturer or transporter. Consumers are not likely to
come in contact with the undiluted substance. Hydrochloric acid exists naturally as a major
component of gastric acid, maintaining a pH of 1-2 in the stomach. For more information on health
hazards and recommended protective equipment, please refer to the (M)SDS.
Exposures may affect human health as follows:
Effect Assessment
Acute Toxicity
Result
Inhalation: May cause respiratory irritation. In serious cases, can
lead to pulmonary edema and death (from Eurochlor website).
Irritation
Causes severe skin burns. May be corrosive.
Causes eye burns. May be corrosive.
Sensitization
Based on available data, not sensitizing.
Mutagenicity
Based on available data, not classified.
Carcinogenicity
Based on available data, not considered carcinogenic.
Toxicity after repeated exposure Effects from repeated exposure may cause altered lung function
or difficulty breathing.
Toxicity for reproduction
Based on available data, not classified as a reproductive or
developmental toxicant.
Environmental Information:
Note: The information in this chapter is intended to provide brief and general information of this
substance’s environmental impact. The results in the table below refer to testing performed with the
non formulated, undiluted substance. The data do not replace the data given in the (M)SDS. For
more information and recommended protective measures please refer to the (M)SDS.
Effect Assessment
Aquatic Toxicity
Persistence and degradability
Bioaccumulation potential
Result
Not toxic to aquatic life, however decreasing the pH in
waterways can lead to adverse effects.
As the substance is inorganic, it is regarded as not
biologically degradable or persistent.
Not expected to bioaccumulate.
Risk Management
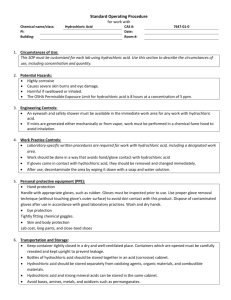
Workplace Management: Risk management measures for industrial site use include containment
through engineering controls and personal protective equipment. Use appropriate respiratory
protection since there is the opportunity for exposure to anhydrous Hydrochloric Acid.
DuPont™ Hydrochloric Acid
Page 3 of 4
Revised September 14, 2012
Uses advised against are those that may lead to aerosol formation (mists), which could be harmful
for the eyes, skin and respiratory system.
Workers should follow the recommended safety measures contained within the (Material) Safety
Data Sheet ((M)SDS) and on any product packaging. Employees should be trained in the
appropriate work processes and safety equipment to limit unnecessary exposure to chemical
substances. Occupational use of this substance is considered to be safe provided the recommended
safety measures in the (M)SDS are followed.
Consumer Risk Management: For end-use products containing Hydrochloric acid, such as
household cleaners, consumers should always follow manufacturer's instructions for the safe
handling of the product. Always read and follow the instructions given in the product labels prior to
use.
Regulatory Information:
The information in this summary is basic information and is not intended to provide emergency
response, medical or treatment information. Additional safety and heath information can be found
on the (Material) Safety Data Sheet (M)SDS. Always refer to the (Material) Safety Data Sheet for
guidance on regulatory requirements that may govern the manufacture, sale, transportation, use
and/or disposal of this chemical or product. Regulations may vary by region, country, state, county,
city, or local government.
First Aid Information:
For all First Aid or Emergency information, consult the (Material) Safety Data Sheet ((M)SDS).
Information Sources:
Data is compiled from a variety of sources, including publicly available documents, internal data and
other sources such as, but not limited to, Chemical Safety Reports and (Material) Safety Data Sheets
((M)SDS).
Contact Information:
E.I. du Pont de Nemours and Company, Wilmington, DE 19880
USA Customer Service:
Toll Free: 1-800-774-1000
Global: 1-843-335-5912
Hours: 8:00 a.m. - 7 p.m. EST
Copyright © 2012 DuPont or its affiliates. All rights reserved. The DuPont Oval Logo and DuPont™ are registered
trademarks of E.I. du Pont de Nemours and Company or its affiliates. No part of this material may be reproduced, stored
in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or
otherwise without the prior written permission of DuPont.
This document is provided for informational purposes only and is based on technical information, that to the best
knowledge of DuPont on the date issued, is believed to be reliable. This document refers only to the specific material
named and does not relate to its use in combination with any other material or process. This document is provided at no
charge and accordingly, no warranties of any kind, express or implied, are made regarding the technical data and
information provided. Furthermore, DuPont assumes no liability or obligation in connection with use of this
information. To obtain the most accurate and current information, consult the appropriate Safety Data Sheet (SDS) prior
to use of the material named herein. DuPont reserves the right to amend and update this information at any time.
DuPont™ Hydrochloric Acid
Page 4 of 4
Revised September 14, 2012