CHEM 1310: General Chemistry Sections L & M EXAM #1 Study Guide
advertisement

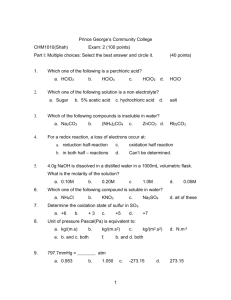
CHEM 1310: General Chemistry Sections L & M EXAM #1 Study Guide (covering Chemical Principles Chapters 1-5) Chapter 2: Atoms, Molecules and Ions Know composition of an atom: protons, neutrons, electrons Know charges of atomic particles Know what an isotope is and how it is designated (Section 2.6) in terms of atomic number and mass number Know group names in Periodic Table of the Elements and how to interpret information from the Periodic Table Know names of ions and how to name simple compounds (Section 2.9, especially tables) Chapter 3: Stoichiometry Balance a chemical equation Calculate moles, # molecules, mass, Molar mass, etc. Calculate % composition of compounds Determine the empirical formula or molecular formula of a compound Deduce relationships from a balanced chemical such as shown in Table 3.2 Calculate amount of reactants or products from a balanced chemical equation Deduce the limiting reactant Calculate theoretical yield and percent yield Chapter 4: Types of Chemical Reactions and Solution Stoichiometry Polarity of water Definition of an electrolyte (and a nonelectrolyte) and what characterizes a strong vs. weak electrolyte Calculations involving molarity and dilutions Predict precipitate that would form from a precipitation reaction (Table 4.1) Write balanced chemical equations that describe a precipitation reaction Calculate amount of base needed to titrate a specific acid and vice versa. Identify oxidizing agent and reducing agent in a redox reaction. Identify oxidation half reaction and reduction half reaction. Balance redox reactions Deduce oxidation state (or oxidation number) for a given element in a compound. Chapter 5: Gases Boyle’s Law and calculations involving it Charles’s Law and calculations involving it Ideal Gas Law and calculations involving it STP Calculate molar mass of a gas from the ideal gas law Dalton’s Law of Partial Pressures and calculations involving it Average kinetic energy of a gas Average root mean square velocity of a gas Definition of effusion and diffusion