Qualitative Analysis of Metallic Elements Solubility Rules
advertisement

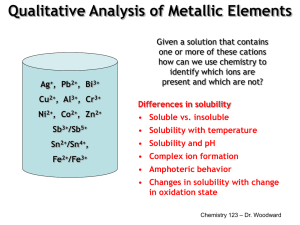
Qualitative Analysis of Metallic Elements Ag+, Pb2+, Bi3+ Cu2+, Al3+, Cr3+ Ni2+, Co2+, Zn2+ Given a solution that contains one or more of these cations how can we use chemistry to identify which ions are present and which are not? Differences in solubility • Soluble vs. insoluble Sb3+/Sb5+ • Solubility with temperature Sn2+/Sn4+, • Solubility and pH Fe2+/Fe3+ • Complex ion formation • Amphoteric behavior • Changes in solubility with change in oxidation state Chemistry 123 – Dr. Woodward Solubility Rules Soluble vs. Insoluble is not very helpful. It can really only be used to separate Ag+ and Pb2+ from the other ions. Chemistry 123 – Dr. Woodward 1 Ag+, Pb2+, Bi3+, Cu2+, Sb3+, Sb5+, Sn2+, Sn4+, Fe2+, Fe3+, Al3+, Cr3+, Ni2+, Co2+, Zn2+ Separation into Groups cold dilute HCl AgCl, PbCl2 Remaining Cations & Pb2+ Group I – Insoluble chlorides H2S (+ HNO3) PbS, Bi2S3, CuS, Sb2S5, SnS2 Group II – Acid insoluble sulfides Group III– Base insoluble sulfides & hydroxides Remaining Cations 1. NH4Cl, NH3 2. H2S NiS, CoS, ZnS, Fe(OH)3, Al(OH)3, Cr(OH)3 Groups 4 & 5 Chemistry 123 – Dr. Woodward Group I – Insoluble chlorides AgCl(s) (s) ↔ Ag+(aq) (aq) + Cl−(aq) (aq) Ksp = 1.8 × 10−10 PbCl2(s) ↔ Pb2+(aq) + 2Cl−(aq) Ksp = 1.7 × 10−5 • AgCl & PbCl2 are both white solids. • Dilute HCl is used rather than a chloride salt (i.e. NaCl) to avoid precipitating SbOCl and BiOCl (which are soluble in acidic solutions). • If the chloride concentration is too high (conc. HCl) complex ion formation, AgCl2− & PbCl42−, causes the precipitates to dissolve. • Because PbCl2 is only moderately insoluble adding HCl does not completely remove Pb2+ from the mixture. So we need to consider Pb2+ once again with the group cations. Chemistry 123II – Dr. Woodward 2 Separating and Confirming Pb2+ • Because the solubility of PbCl2 increases considerably upon heating (6.73 g/L at 0 °C vs. 33.4 g/L at 100 °C) we can dissolve PbCl2 from AgCl by heating. • To confirm the presence of lead we add potassium dichromate to precipitate PbCrO4 which is a distinctive orange-yellow solid Favored for low pH (acidic) Favored for high pH (basic) Cr2O72−(aq) + H2O(l) ↔ 2CrO42−(aq) + 2H+(aq) orange yellow Ksp = 1.8 × 10−14 Pb2+(aq) + CrO42−(aq) ↔ PbCrO4(s) orangeorange-yellow Chemistry 123 – Dr. Woodward Confirming Ag+ After separating Pb2+ we should have a white precipitate of AgCl. How can we make sure the precipitate is AgCl and not undissolved PbCl2? Concentrated NH3 should dissolve the precipitate due to complex ion formation AgCl(s) (s) + 2NH3(aq) ↔ Ag(NH3)+(aq) + Cl−(aq) (aq) If Pb2+ remains in solution the white precipitate Pb(OH)2 will form on making the solution basic. To confirm Ag+ we reverse this by making the sol’n acidic Ag(NH3)+(aq) + Cl−(aq) (aq) + 2H+(aq) ↔ AgCl(s) (s) + 2NH4+(aq) Chemistry 123 – Dr. Woodward 3 Separation into Groups Ag+, Pb2+, Bi3+, Cu2+, Sb3+, Sb5+, Sn2+, Sn4+, Fe2+, Fe3+, Al3+, Cr3+, Ni2+, Co2+, Zn2+ cold dilute HCl AgCl, PbCl2 Remaining Cations & Pb2+ Group I – Insoluble chlorides H2S (+ HNO3) Acidic solution Basic (buffered) solution PbS, Bi2S3, CuS, Sb2S5, SnS2 Group II – Acid insoluble sulfides Group III– Base insoluble sulfides & hydroxides Remaining Cations 1. NH4Cl, NH3 2. H2S NiS, CoS, ZnS, Fe(OH)3, Al(OH)3, Cr(OH)3 Groups 4 & 5 Chemistry 123 – Dr. Woodward Aqueous Chemistry of S2H2S(aq) ↔ H+(aq) (aq) + HS−(aq) (aq) Ka1 = 9.5 × 10−8 HS−(aq) (aq) ↔ H+(aq) (aq) + S2−(aq) Ka2 = 1 × 10−19 Because Ka2 is so small there are almost no S2− ions in solution. We can neglect the second reaction. Formation of metal sulfides occurs through reaction of HS− with metal cations, and the concentration of HS− is dependent on the pH. Consider the precipitation of CoS. H2S(aq) ↔ H+(aq) (aq) + HS−(aq) (aq) Ka1 = 9.5 × 10−8 Co2+(aq) + HS−(aq) (aq) ↔ H+(aq) (aq) + CoS(s) (s) 1/Ksp = 2 × 10+21 Co2+(aq) + H2S(aq) ↔ 2H+(aq) + CoS(s) (s) K = 1.9 × 10+14 Chemistry 123 Dr. Woodward Increasing [H+] (lowering pH) drives the equilibrium to –the left. 4 Separation of Group II from Group III Group II Cations Pb2+(aq) + 2HS−(aq) ↔ PbS(s) (s) + H+(aq) (aq) Ksp = 3 × 10−28 Cu2+(aq) + 2HS−(aq) ↔ CuS(s) (s) + H+(aq) (aq) Ksp = 6 × 10−37 Group III Cations Ni2+(aq) + 2HS−(aq) ↔ NiS(s) (s) + H+(aq) (aq) Ksp = 3 × 10−20 Co2+(aq) + 2HS−(aq) ↔ CoS(s) (s) + H+(aq) (aq) Ksp = 6 × 10−22 By making the solution acidic we shift the equilibrium to the left (favors reactants) which makes the Group III Chemistry 123 – Dr. Woodward sulfides dissolve, but not the Group II sulfides. Group II – Acid Insoluble Sulfides Pb2+(aq) + 2HS−(aq) ↔ PbS(s) (s) + H+(aq) (aq) Black ppt 2Bi3+(aq) + 3HS−(aq) ↔ Bi2S3(s) + 3H+(aq) Dk. Brown ppt Cu2+(aq) + HS−(aq) (aq) ↔ CuS(s) (s) + H+(aq) (aq) Black ppt SnCl62−(aq) + 2HS−(aq) ↔ SnS2(s) + 4H+(aq) + 6Cl−(aq) Yellow ppt 2SbCl6−(aq) + 5HS−(aq) ↔ Sb2S5(s) + 5H+(aq) + 12Cl−(aq) Orange ppt Chemistry 123 – Dr. Woodward 5 Pb2+, Bi3+, Cu2+, Sb3+, Sb5+, Sn2+, Sn4+ HNO3 acts as an oxidizing agent Cl− acts as a complexing agent HNO3 + HCl (Aqua regia) Pb2+, Bi3+, Cu2+, SbCl61−, SnCl62− CH3CSNH2 (thioacetamide) decomposes on heating to give ~0.10 M H2S(aq) Removes excess acid, be careful not to overdo it. Evaporate to a paste HNO3 CH3CSNH2, heat PbS (black), Bi2S3 (dark brown), CuS (black), Sb2S5 (orange), SnS2 (yellow) NaOH Copper subgroup PbS, Bi2S3, CuS SnS2 & Sb2S5 are amphoteric Antimony subgroup SbS4 3−, 3−, SbO4 123 SnS– 4Dr.,Woodward SnO4 Chemistry 3− 3− 6