ees4201 - water chemistry - UF Connect
advertisement
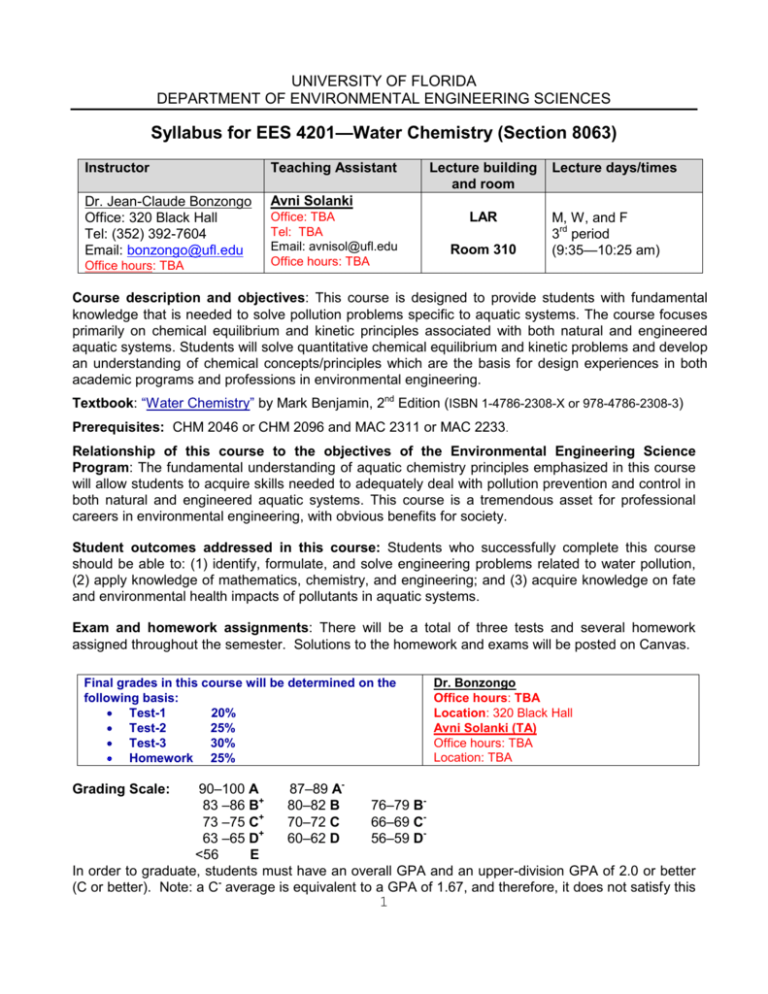
UNIVERSITY OF FLORIDA DEPARTMENT OF ENVIRONMENTAL ENGINEERING SCIENCES Syllabus for EES 4201—Water Chemistry (Section 8063) Instructor Teaching Assistant Dr. Jean-Claude Bonzongo Office: 320 Black Hall Tel: (352) 392-7604 Email: bonzongo@ufl.edu Avni Solanki Office hours: TBA Office: TBA Tel: TBA Email: avnisol@ufl.edu Office hours: TBA Lecture building and room LAR Room 310 Lecture days/times M, W, and F 3rd period (9:35—10:25 am) Course description and objectives: This course is designed to provide students with fundamental knowledge that is needed to solve pollution problems specific to aquatic systems. The course focuses primarily on chemical equilibrium and kinetic principles associated with both natural and engineered aquatic systems. Students will solve quantitative chemical equilibrium and kinetic problems and develop an understanding of chemical concepts/principles which are the basis for design experiences in both academic programs and professions in environmental engineering. Textbook: “Water Chemistry” by Mark Benjamin, 2nd Edition (ISBN 1-4786-2308-X or 978-4786-2308-3) Prerequisites: CHM 2046 or CHM 2096 and MAC 2311 or MAC 2233. Relationship of this course to the objectives of the Environmental Engineering Science Program: The fundamental understanding of aquatic chemistry principles emphasized in this course will allow students to acquire skills needed to adequately deal with pollution prevention and control in both natural and engineered aquatic systems. This course is a tremendous asset for professional careers in environmental engineering, with obvious benefits for society. Student outcomes addressed in this course: Students who successfully complete this course should be able to: (1) identify, formulate, and solve engineering problems related to water pollution, (2) apply knowledge of mathematics, chemistry, and engineering; and (3) acquire knowledge on fate and environmental health impacts of pollutants in aquatic systems. Exam and homework assignments: There will be a total of three tests and several homework assigned throughout the semester. Solutions to the homework and exams will be posted on Canvas. Final grades in this course will be determined on the following basis: Test-1 20% Test-2 25% Test-3 30% Homework 25% Dr. Bonzongo Office hours: TBA Location: 320 Black Hall Avni Solanki (TA) Office hours: TBA Location: TBA 90–100 A 87–89 A83 –86 B+ 80–82 B 76–79 B+ 73 –75 C 70–72 C 66–69 C+ 63 –65 D 60–62 D 56–59 D<56 E In order to graduate, students must have an overall GPA and an upper-division GPA of 2.0 or better (C or better). Note: a C- average is equivalent to a GPA of 1.67, and therefore, it does not satisfy this Grading Scale: 1 graduation requirement. For more information on grades and grading policies, please visit: https://catalog.ufl.edu/ugrad/current/regulations/info/grades.aspx Class Attendance: Students are encouraged to attend all classes. History indicates that students who do not attend class do poorly on examinations and suffer the consequence of low grades. Canvas: We will use Sakai for this course. Notes consisting of power point slides to be used in class, homework assignments and other information will be posted on UF-Canvas site. Online information regarding common tools in Sakai is provided for students. This information is available via the Student Intro to ELS link at http://lss.at.ufl.edu. Students needing assistance with the computer and technical requirements for using E-learning, should seek this assistance from the UF HelpDesk http://helpdesk.ufl.edu/ 352-392-4357, helpdesk@ufl.edu). The HelpDesk can also assist students who are having trouble logging into E-learning. Make-up Exam Policy: Make-up exams are only given for medical reasons. A student may request a make-up exam if they cannot attend the scheduled exam for medical reasons and can show proofs of such reasons (e.g. letter from a PCP or hospital). The student must contact the instructor (bonzongo@ufl.edu) before the exam to state that he/she will not be able to attend the exam. Penalties on overdue homework assignments: Overdue assignments will be penalized at a rate of -50 points per day. For instance, a homework assignment due on 10/10/2015 and turned in on 10/11/2015 will be graded over 50 and not 100. Students with Disabilities: Students requesting classroom accommodation must first register with the Dean of Students Office. The Dean of Students Office will provide documentation to the student who must then provide this documentation to the Instructor when requesting accommodation. Cell Phones: Cell phones must be turned off or put in silent mode at the beginning of class; use of cell phones for calls or messaging during class is prohibited. Academic Honesty: All students admitted to the University of Florida have signed a statement of academic honesty committing themselves to be honest in all academic work and understanding that failure to comply with this commitment will result in disciplinary action. This statement is a reminder to uphold your obligation as a UF student and to be honest in all work submitted and exams taken in this course and all others. Software Use: All faculty, staff, and students of the University of Florida are required and expected to obey the laws and legal agreements governing software use. Failure to do so can lead to monetary damages and/or criminal penalties for the individual violator. Because such violations are also against University policies and rules, disciplinary action will be taken as appropriate. UF Counseling Services: Resources are available on-campus for students having personal problems or lacking clear career and academic goals. The resources include: Counseling and Wellness Center, 3190 Radio Rd., 392-1575, Personal and Career Counseling. SHCC Mental Health, http://shcc.ufl.edu/,Student Health Care Center, 392-1161, Personal Counseling. Center for Sexual Assault/Abuse Recovery and Education (CARE), Student Health Care Center, 392-1161, sexual assault counseling. Career Resource Center, Reitz Union, 392-1601, career development assistance and counseling. 2 TENTATIVE LECTURE SCHEDULE Week Dates Lecture Topics Basics of Aquatic Chemistry 8/24 8/26 1 8/28 2 8/31 9/02 9/04 The water molecule Dissolution of solutes in water Quantitation in water chemistry Chemical Reactivity, Reactions & Equilibrium Chemical reactions Activity and Activity Coefficients Chemical Equilibrium Kinetics-Based Interpretation of Equilibrium 3 9/07 NO CLASS 9/09 9/11 Reaction Kinetics and Rate Constants Effect of Temperature Order of reactions Thermodynamics & Water Chemistry 4 9/14 9/16 9/18 Gibbs free energy of reaction under equilibrium and non-equilibrium conditions Saturation Index Precipitation/Dissolution reactions Acids and Bases – PARTS 1 & 2 5 6 7 9/21 9/23 9/25 9/28 9/30 10/02 10/05 10/07 10/09 8 10/12 10/14 10/16 9 10/19 10/21 10/23 Structure of acids & bases Acid/Base Speciation vs. pH Log C – pH diagrams Solving Acid/Base problems The mass balance on H Identifying dominant species in acid-base systems Geochemical Equilibrium Models MINTEQ Using chemical equilibrium software to develop Log C – pH diagrams Titrations and Buffers Qualitative & quantitative considerations Data interpretation Titrations, Equivalence Points Alkalinity and acidity Using MINTEQ to simulate titrations 3 Textbook Assignments Chap/pages 1 pp: 2-30 HW-1 8/28 2 pp: 38-73 HW-2 9/04 3 pp: 78-113 HW-3 9/11 None Handout TEST-1 9/18 4&5 Part-1: Chap-4 pp: 217-280 Part-2: Chap-5 pp: 292-341 HW-4 10/02 7 pp: 354-375 8 pp: 378-453 HW-5 10/16 10 10/26 10/28 10/30 Gas/Liquid Equilibrium Gas–Liquid Equilibrium and Henry’s Constant Effect of Ionization on Gas–Liquid Equilibria CO2 Dissolution and Alkalinity/Acidity of H2O Chemistry of metals in aquatic systems 11/02 11/04 11 HOMECOM ING – NO CLASS on Friday 11/06 12 11/09 NO CLASS ON 11/11 11/13 13 14 11/16 11/18 11/20 11/23 11/25 11/27 15 16 12/07 12/09 pp: 378-453 TEST-2 10/30 10 & 11 Solution Phase Reactions Metal oxidation states Aquo-complexes of metals Log C – pH diagram for metals Metal speciation is systems with ligands Predominance phase diagrams pp: 528-566 Precipitation & Dissolution Reactions Formation of solids containing metal ions The chemical activity of pure solids pp: 576-647 The solubility products Non-hydroxo-complexes in systems with solids MINETEQ Redox Chemistry Definitions (oxidation, reduction, reduction, oxidation sate) Definitions of e0 and pe0 - the EH scale Oxidation and reduction of water Biochemical Oxygen Demand (BOD) Redox titrations, redox buffering Geochemical redox sequence pe – pH phase diagrams: Distribution of species in aquatic systems HW-7 11/03 12 pp: 663-756 HW-8 11/23 THANKSGIVING – NO CLASS Adsorption Reactions 11/30 12/02 12/04 9 13 Adsorption Terminology & Mechanisms Adsorption Isotherms Modeling Adsorption with MINTEQ Electrical Potential & Adsorption pp: 770-840 There will be no final exam during the final exam week. 4 TEST-3 12/09







