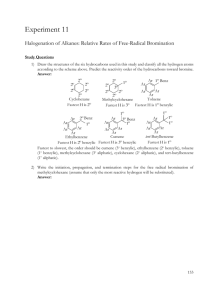
Stereochemistry of the Addition of Bromine to trans
advertisement
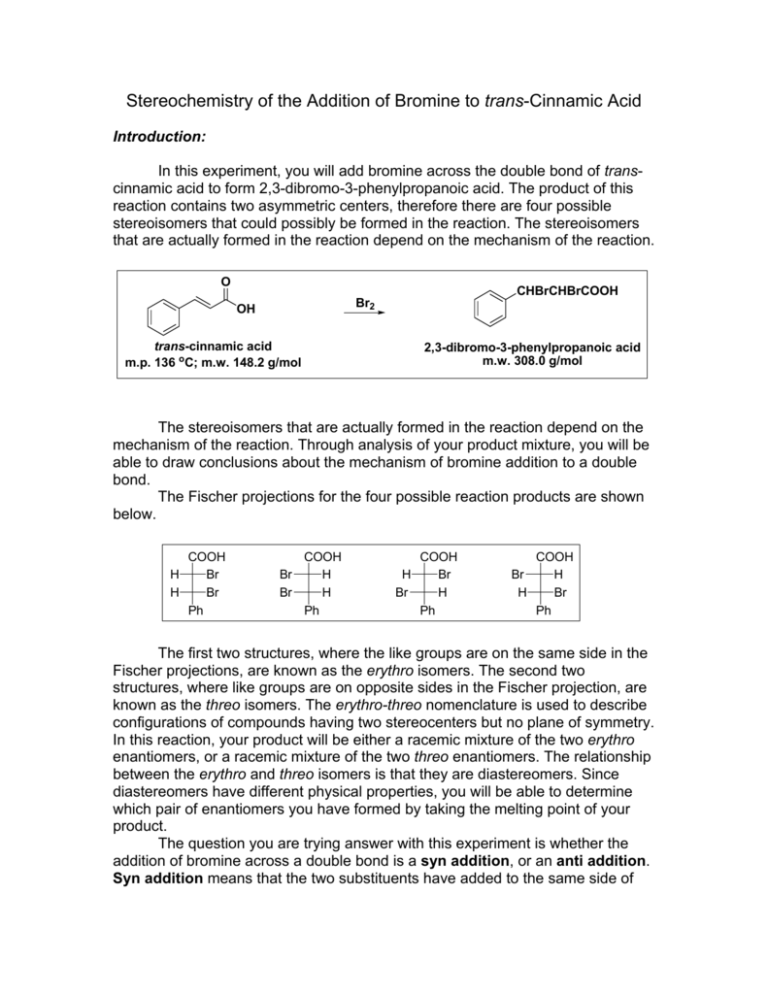
Stereochemistry of the Addition of Bromine to trans-Cinnamic Acid Introduction: In this experiment, you will add bromine across the double bond of transcinnamic acid to form 2,3-dibromo-3-phenylpropanoic acid. The product of this reaction contains two asymmetric centers, therefore there are four possible stereoisomers that could possibly be formed in the reaction. The stereoisomers that are actually formed in the reaction depend on the mechanism of the reaction. O CHBrCHBrCOOH Br2 OH trans-cinnamic acid m.p. 136 oC; m.w. 148.2 g/mol 2,3-dibromo-3-phenylpropanoic acid m.w. 308.0 g/mol The stereoisomers that are actually formed in the reaction depend on the mechanism of the reaction. Through analysis of your product mixture, you will be able to draw conclusions about the mechanism of bromine addition to a double bond. The Fischer projections for the four possible reaction products are shown below. COOH H Br H Br Ph COOH Br H Br H Ph COOH H Br Br H Ph COOH Br H H Br Ph The first two structures, where the like groups are on the same side in the Fischer projections, are known as the erythro isomers. The second two structures, where like groups are on opposite sides in the Fischer projection, are known as the threo isomers. The erythro-threo nomenclature is used to describe configurations of compounds having two stereocenters but no plane of symmetry. In this reaction, your product will be either a racemic mixture of the two erythro enantiomers, or a racemic mixture of the two threo enantiomers. The relationship between the erythro and threo isomers is that they are diastereomers. Since diastereomers have different physical properties, you will be able to determine which pair of enantiomers you have formed by taking the melting point of your product. The question you are trying answer with this experiment is whether the addition of bromine across a double bond is a syn addition, or an anti addition. Syn addition means that the two substituents have added to the same side of the double bond, anti addition means that the two substituents have added to opposite sides of the double bond. Procedure: To a medium test tube add 150 mg of trans-cinnamic acid and 0.6 mL of glacial acetic acid. Place the test tube in a 50 oC water bath until all the transcinnamic acid is dissolved. Add 1.0 mL of a 1.0 M bromine in acetic acid solution to the test tube. Add the solution dropwise over a period of not less than 10 min. Failure to do so will usually result in a failed reaction. Continue heating at 50 oC until the red-brown color of the bromine fades to light orange, then continue to heat for 15 minutes more. If the mixture becomes colorless (or nearly so) during this period, add more of the bromine solution dropwise until color just persists. If the mixture has a distinct orange color at the end of the reaction period, add a drop or two of cyclohexene to turn it light yellow. Cool the reaction mixture in an ice-water bath for 10 minutes or more, scratching the sides of the vial to induce crystallization if necessary. Collect the product by vacuum filtration on a Buchner funnel and wash the solid with several portions of ice-cold water, until the acetic acid odor is hardly noticeable. Purify the product by recrystallization from 50% ethanol. Dry the product, take the weight and measure the melting point to determine whether you have the erythro (m.p. 204 oC) or threo isomer (m.p. 95 oC). Safety Considerations: • Take care when using the concentrated acid or the bromine solution to not get it on your skin or clothing. • There will be a waste container for organic liquid waste • Dispose of used glass pipettes in the broken glass container Data, Calculations, and Discussion: • Obtain the mass of the 2,3-dibromo-3-phenylpropanoic acid product and calculate theoretical and % yields. • Record the melting point of the product and use it to determine which pair of enantiomers, the erythro or the threo, you formed. • From the isomer formed, decide whether the addition was a syn addition or an anti addition. Be careful in deciding this as it may not be as obvious as it seems from looking at the Fischer projections. Remember that carbon-carbon single bonds are freely rotating, and that Fischer projections show the stereoisomers in their eclipsed conformations. You should redraw them as skeletal structures or perspective formulas in order be to able to clearly see whether the two bromines were added to the same side or opposite sides of the double bond. • Draw and name (using R and S designations) the two enantiomers that you formed in the reaction.