Answers to Chapter 5
advertisement

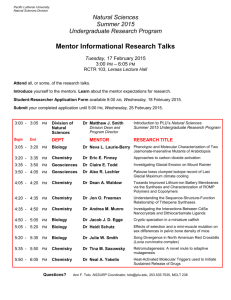
Introduction to Bioorganic Chemistry and Chemical Biology Answers to Chapter 5 (in-text & asterisked problems) Answer 5.1 H27 PDB 3ZNF C8 C5 H21 Introduction to Bioorganic Chemistry and Chemical Biology | A5110 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 5.2 design by www.blink.biz © www.garlandscience.com +H 2N NH2 NH IRGERA N H O H N N H O 2N O O H N N H O NH +H O H N O O+H NH2 NH2 2N NH ent-IRGERA N H O H N N H O 2N O O H N N H O NH +H O H N O O+H NH2 NH2 2N NH O AREGRI O O H N N H N H O NH +H 2N NH2 O H N O O O- N H H N 1 2 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 +H NH2 2N NH O ent-AREGRI N H O O H N N H O NH this “retro-inverso“ peptide is the best match of the natural sequence IRGERA +H 2N O H N O O N H H N O- NH2 Answer 5.3 A For NKDVLRRMKK: Residue Charge Arg (R) +1 × 2 Lys (K) +1 × 3 +H N terminus 3 +1 × 1 Glu (E) –1 × 0 Asp (D) –1 × 1 –O –1 × 1 2C– terminus Introduction to Bioorganic Chemistry and Chemical Biology | A5111 Net charge +4 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz Similar calculations for RFRGDYFAK and KGNIDKFTEK yield net charges of +2 and +1, respectively. BThe peptide is expected to bind better to a negatively charged surface through ionic interactions, because opposite charges attract. Answer 5.4 Residues in the peptide are labeled in red; residues in MDM2 are labeled in blue. L26 V99 M62 W23 L54 F19 I61 I99 F91 L57 Introduction to Bioorganic Chemistry and Chemical Biology | A5112 Van Vranken5.5 & Weiss | 978-0-8153-4214-4 Answer © www.garlandscience.com design by www.blink.biz H383, H387, and E411. H383 E411 H387 Introduction to Bioorganic Chemistry and Chemical Biology | A5113 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 Answer 5.6 + O O O O: OH+ H A OH -A: HO H HO + HO Introduction to Bioorganic Chemistry and Chemical Biology | A5114 Van Vranken5.7 & Weiss | 978-0-8153-4214-4 Answer © www.garlandscience.com design by O www.blink.biz HN N O O O O O N N H OH Fmoc-PNA-T-OH Introduction to Bioorganic Chemistry and Chemical Biology | A5115 Van Vranken5.8 & Weiss | 978-0-8153-4214-4 Answer © www.garlandscience.com design by www.blink.biz Pro and Gly prefer turns Val, Ile, and Tyr prefer sheets HO O NH O H N N H OEt O N H Et O H N N O O N H N O O NH H N O OH Introduction to Bioorganic Chemistry and Chemical Biology | A5116 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 5.9 © www.garlandscience.com design by www.blink.biz A A1,3 strain is most important for ϕ angles; A1,2 strain is most important for ψ angles. B C CONH side chain N H H side chain O CONH O H side chain N H H N H = 0° = 160° CONH Introduction to Bioorganic Chemistry and Chemical Biology | A5117 Van Vranken & Weiss | 978-0-8153-4214-4 side chain O © www.garlandscience.com design by www.blink.biz H N H = 0° CONH = 160° duction to Bioorganic Chemistry and Chemical Biology | A5117 Answer 5.10 ranken & Weiss | 978-0-8153-4214-4 w.garlandscience.com design by www.blink.biz - O: + S: + H A OH +S : + A- HS .. R -A : + OH S: S 2 R + S: S .. R SH R A H OH S: S H + R R + S R S H -A: Introduction to Bioorganic Chemistry and Chemical Biology | A5118 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz R S S R : OH S: S R 3 4 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 Answer 5.11 Cys R + Cys-S P R R : PR 3 S S Cys R Cys-S P O RR H R .. Cys-S P O RR :B R H Cys-S P O + RR H : OH2 Cys-S: :B Cys-SH H B R R P O R Introduction to Bioorganic Chemistry and Chemical Biology | A5119 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 5.12 © www.garlandscience.com design by www.blink.biz There are seven WD domains in each half of the dimer, leading to a total of 14 WD domains. Answer 5.13 A H A .. HN +H 2N R 3N N H + H R R N + H R R R N R B -A: enamine R R NH .. R +H + H N H R 3N : A- R 2N H R ..NH2 H R NH .. 2 +H NH2+ -A: A H R R NH imine : A- H A .. H2N R : A- R N: H R R .. NH2 R +H H2N R NH2+ R H2N A H R NH R NH2 R H2..N aldol cross-link R *Answer 5.14 Introduction to Bioorganic Chemistry and Chemical Biology | A5120 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz NH3+ NH3+ spinorphin: LVVYPWT NH3+ O H2+ O N O H N N H H N N H O O H N N H O +H O 3N O H N N H O- OH O N N H O NH N H O O H N O- O HO O NH NH3+ NH2+ H2N SV40 NLS sequence: PKKKRKV N H OH NH2 O HN O +H 3N N H S H N O O N H H N O O O N H O NH2 N O N H H N O O O O O- H N N H O O N S HN H O HN O S N OO H N NH O O oxytocin: CYIQNCPLG Introduction to Bioorganic Chemistry and Chemical Biology | A5121 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz O O N H H HO S phakellistatin 13: cyclo-[FGPTLWP] H N N malformin A: cyclo-[D C D CV D LI] Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 *Answer 5.15 A substance P RPKPQQFFGLM B antimicrobial peptide cyclo-[RRWWRF] or cyclo-[RWWRFR] or cyclo-[WWRFRR] or cyclo-[WRFRRW] or cyclo-[RFRRWW] or cyclo-[FRRWWR] or cyclo-[RRWWRF] C synthetic integrin antagonist CWLDVC *Answer 5.17 A Residue Charge Arg (R) +1 × 0 Introduction Lys (K)to Bioorganic Chemistry +1 ×and 0 Chemical Biology | A5122 Van Vranken & Weiss | 978-0-8153-4214-4 design by www.blink.biz © www.garlandscience.com +H N terminus +1 × 1 3 Glu (E) –1 × 0 Asp (D) –1 × 5 –O –1 × 1 2C terminus Net charge –5 Residue Charge Arg (R) +1 × 6 Lys (K) +1 × 2 +H +1 × 1 B 3N terminus Glu (E) –1 × 0 Asp (D) –1 × 0 –O –1 × 0 2C terminus Net charge +9 5 6 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 *Answer 5.19 :B H S O N H O O N H HN .. -O S O N + H2 .. SO HN H B+ O HN .. O S O O H2N HN B H O -O S .. N H O HN .. -S HS O H2N O H2N HN HN Introduction to Bioorganic Chemistry and Chemical Biology | A5125 Van Vranken & Weiss | 978-0-8153-4214-4 *Answer 5.23 design by www.blink.biz © www.garlandscience.com O: Asp H A CF 3 O O O O Asp OH+ R CF 3 + O O O H CF 3 OH A H NO : O Asp + O O: HO : A- Introduction to Bioorganic Chemistry and Chemical Biology | A5129 Van Vranken & Weiss | 978-0-8153-4214-4 design by www.blink.biz *Answer 5.24 © www.garlandscience.com Trp O O: Asp H A OH+ Asp OH Asp Trp -A: O O + Introduction to Bioorganic Chemistry and Chemical Biology | A5130 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz N H H Trp + N H N H Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 7 *Answer 5.26 A Initial protonation occurs on the amide carbonyl, not the amide nitrogen. A Initial protonation occurs on the amide carbonyl, not the amide nitrogen. H A .. H A .. O Ph Ph O Ph Ph N Ph R R N Ph H H H H Si i-Pr i-Pr Si i-Pr i-Pr i-Pr .. i-Pr .. O O O O- OH++ Ph OH Ph R R CF 3 CF 3 Ph Ph Ph Ph N N H H H H i-Pr - Si i-Pr Si CF 3COO CF 3COO OH OH + NH + NH R R Ph + Ph Ph + Ph Ph i-Pr Ph i-Pr i-Pr i-Pr H O H O OH OH +CPh3 +CPh3 R R i-Pr i-Pr i-Pr i-Pr Si i-Pr Si i-Pr CF 3COO CF 3COO NH .. NH .. H A H A R R NH2++ NH2 : A: A- O O R R NH2 NH2 Ph Ph Ph Ph Ph H Ph H B Protonation of N would disrupt the aromaticity of the imidazole ring. B Protonation of N would disrupt the aromaticity of the imidazole ring. Therefore initial protonation occurs on the N not N . Therefore initial protonation occurs on the N not N . Ph Ph Ph Ph Ph Ph Ph Ph H A Ph Ph H A .. Ph Ph .. N N N N N N R R N .. N .. C C R R D D R R N N H H R R H A H A H A H A .. .. O O R R R R N N H H O O A H OH A H OH R .. .. R N O N O H H H H i-Pr Si i-Pr Si CF 3COO CF 3COO i-Pr i-Pr i-Pr i-Pr R R N N H H H H +O +O H A H A .. .. O O R R N+ N H+ H R R OH++ OH OH OH R R N N H H O O H H i-Pr - Si i-Pr Si CF 3COO CF 3COO H+ H N+ N + + OH OH O O N N H H H H i-Pr - Si i-Pr Si CF 3COO CF 3COO + + + + + + H O H O : A- O:: H : A-O H R R R .. .. R N R N R NH2 O O N N NH2 H2++ O H2++ O H2 H2 a concerted, one-step E2 mechanism is also plausible a concerted, one-step E2 mechanism is also plausible A A + CO2 + CO2 + + i-Pr3SiO 2CCF 3 i-Pr3SiO 2CCF 3 + Me3C-H + Me3C-H E Note that protonation of thioethers is extremely unfavorable (pKa' < -8). E Note that protonation of thioethers is extremely unfavorable (pKa' < -8). Cys Cys H A H A Ph Ph Ph Ph Ph Ph .. .. S S H H i-Pr - Si i-Pr Si CF 3COO CF 3COO i-Pr i-Pr i-Pr i-Pr Cys Cys Ph + Ph Ph + Ph Ph Ph H +H +S S Ph Ph Ph Ph Ph Ph i-Pr3SiO 2CCF 3 i-Pr3SiO 2CCF 3 Cys Cys SH SH + Ph3C-H + Ph3C-H Introduction to Bioorganic Chemistry and Chemical Biology | A5132 Introduction Bioorganic Chemistry and Chemical Biology | A5132 Van Vranken &to Weiss | 978-0-8153-4214-4 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz © www.garlandscience.com design by www.blink.biz + + +CPh3 +CPh3 Ph + Ph Ph + Ph Ph i-Pr Ph i-Pr i-Pr i-Pr i-Pr3SiO 2CCF 3 i-Pr3SiO 2CCF 3 + Ph3C-H + Ph3C-H i-Pr3SiO 2CCF 3 i-Pr3SiO 2CCF 3 + Ph3C-H + Ph3C-H + + i-Pr i-Pr i-Pr i-Pr 8 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 *Answer 5.28 A KVKVKVKVKVKVK, because β-branched amino acids have high β-sheet propensities and low α-helix and turn propensities. B GPPGPPGPPGPPGPPGPPGPP, because Pro has low α-helix and β-sheet propensities. CNLEDKAEELLSKNYHLENEVARL, because it has a leucine zipper motif with Leu at every seven residues. D GPPGPPGPPGPPGPPGPPGPP, because (GPP)n forms a collagen triple helix. E GPPGPPGPPGPPGPPGPPGPP, because Pro has low α-helix and β-sheet propensities. *Answer 5.31 AThe calcium-binding loops are underlined: MADQLTEEQIAEFKEAFSLFDKDGDGTITTKELGTVMRSLGQNPTEAELQDMINEVDADGNGTIDFPEFLTMMARKMKDTDSEEEIREAFRVFDKDGNGYISAAELRHVMTNLGEKLTDEEVDEMIREADIDGDGQVNYEEFVQMMTAK BThe first calcium-binding loop is positions 21–32, -DKDGDGTITTKE-. The first and last positions of the calcium-binding loop are the only positions conserved as anionic amino acids. Therefore Asp21 and Glu32 are most likely to bind as anionic carboxylates. In the crystal structure for PDB 1CFC, both of the side-chain carboxylate oxygens of Glu32 bind to the calcium ion. Because asparagines are also tolerated at the second and third ligand positions, Asp23 and Asp25 probably coordinate as neutral ligands through the side-chain carbonyls. Because phenylalanine is also tolerated at the fourth ligand position, it is likely that the backbone carbonyl, and not the side chain, is coordinating to calcium. Because serine is a common ligand for the fifth position, Thr29 probably binds through the side-chain hydroxyl. As a result of the high pKa of alcohols compared with carboxylic acids, Thr29 probably binds as a neutral OH ligand. Asp25 H N O Thr27 HN Asp23 O O OH HN OH O Ca O O NH OH O O Asp21 OH NH O CH3 Thr29 O O HN NH Glu32 O Introduction to Bioorganic Chemistry and Chemical Biology | A5135 Vrankenis& Weiss | 978-0-8153-4214-4 C Van Glycine highly flexible and can adopt tight turns. Isoleucine and valine are © www.garlandscience.com design by www.blink.biz β-branched hydrophobic amino acids that limit the flexibility of the side chain and loop. *Answer 5.32 ATwo types of dimers are known. The dimer based on swapping the S-peptide (PDB 1BSR) has the S-peptide from the first molecule bound in the cleft of the second molecule, and vice versa. domain-swapped dimer domain-swapped dimer Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 BThere may be many solutions to this problem. Any mutation to the loop that prevents the helix from binding to the cleft in residues 1–113 will favor the formation of the domain-swapped dimer. Investigators have induced dimerization by generating a deletion variant Δ(114:119) that lacks the flexible loop. wild type protein engineering (truncate loop) deletion mutant domain-swapped dimer Introduction to Bioorganic Chemistry and Chemical Biology | A5136 Van Vranken & Weiss | 978-0-8153-4214-4 *Answer 5.36 design by www.blink.biz © www.garlandscience.com bonds with limited rotation due to A1,3 strain A 1,3 strain R R' H HO R" O R OH O O OH H R" R' O NH2 OH Introduction to Bioorganic Chemistry and Chemical Biology | A5141 Van Vranken & Weiss | 978-0-8153-4214-4 *Answer 5.38 design by www.blink.biz © www.garlandscience.com A Cys28 makes contacts with DNA (rendered as a brown surface). BThe nearby cysteine residue is Cys120. C CysteineS thiols are Hefficient at conjugate addition reactions. H Cys Cys O S H O H HO HO H O O SO H Cys O to Bioorganic Chemistry and Chemical Biology | A5143 Introduction S Cys Van Vranken & Weiss | 978-0-8153-4214-4 www.garlandscience.com design by www.blink.biz © Introduction to Bioorganic Chemistry and Chemical Biology | A5143 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz 9 10 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 5 *Answer 5.41 resonance-stabilized R R X• H + X H O R • R tautomerization OH R H • O O R O etc • •O O R R H O keto tautomers Introduction to Bioorganic Chemistry and Chemical Biology | A5145 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz R HO enol tautomers (aromatic)