Answers to Chapter 4
advertisement

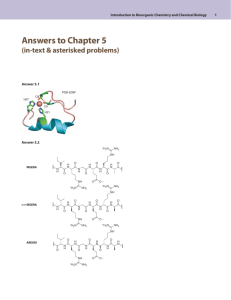
Introduction to Bioorganic Chemistry and Chemical Biology Answers to Chapter 4 (in-text & asterisked problems) Answer 4.1 The bulky tert-butyl group has a strong equatorial preference, the following two conformations will be predominant. The anti isomer cannot hydrolyze through neighboring group participation; the syn isomer can hydrolyze through neighboring group participation. -O OCH3 P O O -O :O - OCH3 P O O .. -O anti syn Introduction to Bioorganic Chemistry and Chemical Biology | A4096 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.2 © www.garlandscience.com design by www.blink.biz There are two plausible mechanisms that avoid expulsion of an anionic H2N– group. Without some mechanistic data, you cannot predict which is the most likely. H B HO .. NH2 B: H O N N HO: N N NH2 R NH3+ R O- -O O- N R N N N B: O H O H B - O .. NH2 NH2 N O- R .. O- N R O H B NH N R O N N R N O :O - NH3+ N R .. O- Introduction to Bioorganic Chemistry and Chemical Biology | A4097 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.3 design by www.blink.biz © www.garlandscience.com A 3' C U C G G GG 5' G A G C CC UG CU C U G U C G 5' C A C A G C 3' AG stem loops U C 3' C U C G G A G CC U G U C G 5' 5' GA G C C GG AG A C A G C 3' UC "kissing complex" Introduction to Bioorganic Chemistry and Chemical Biology | A4098 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz 5' 3' 1 2 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.4 The following figure shows the minimum free-energy structure from RNAfold and GeneBee and the second-lowest energy structure from MC-fold (the lowest-energy structure from MC-fold did not match). G CU A U U U C GG C C G G UG C GC U 3' A C CA U G CGG CUCC A CG G 5' G C C G A G G U G C C U A AA UA CGA G CA C G C U GA U A C G A A G U U G UG Introduction to Bioorganic Chemistry and Chemical Biology | A4099 Van Vranken4.5 & Weiss | 978-0-8153-4214-4 Answer © www.garlandscience.com design by www.blink.biz four types of base pairs: A•T, C•G, G•C, or T•A 5' N N N N N N N N N N 3' N N N N N N N N N four types of unpaired bases: A, C, G, or T N twelve types of mis-matches: A•A, A•C, A•G, C•A, C•C, C•T, G•A, G•G, G•T, T•C, T•G, T•T Total possibilities = 47• 44• 12 = 411• 12 = 50,331,648 Introduction to Bioorganic Chemistry and Chemical Biology | A4100 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.6 www.garlandscience.com design by www.blink.biz © RNA RNA O H :B RNA RNA O .. O O O N O -O .. O N CH3 O O O O - O N .. CH 3 CH3 H B RNA O O .. O Introduction to Bioorganic Chemistry and Chemical Biology | A4101 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz RNA O N+ CH3 H O O H N CH3 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.7 O O Gua Cyt O -O O P O OH O Ade Thy O -O O P O OH O template strand O Ura Ade RNA Pol Mg O O O O P P O O P O -O O O Gua Cyt O -O O P O O HO OH O OH O 5' Gua Cyt O -O Ade Thy P O OH O Ura Ade Cyt Gua Mg O .. 3' OH O Mg O P O + HO OH O O O O P P -O O O O O Cyt O template strand O -O Gua HO 3' OH leaving group O 5' O P O OH O Ade Thy O OH O -O O P O template strand Ura Ade O -O P O Mg O O O P O P O -O O O OH O HO 3' Cyt Gua OH Answer 4.8 Introduction to Bioorganic Chemistry and Chemical Biology | A4102 Van Vranken & Weissare | 978-0-8153-4214-4 Triphosphates highly susceptible to nucleophilic attack, particularly in the presence design by www.blink.biz © www.garlandscience.com 2+ of Mg ions (not shown). The 2ʹ-hydroxyl group of a 3ʹ-nucleotidyl triphosphate would cyclize to give an unstable cyclic 2ʹ,3ʹ-phosphate. HO O Cyt O .O . H O P O -O O P -O O O P OH -O HO O Cyt O O P O O- OH O P -O O O P OH -O Introduction to Bioorganic Chemistry and Chemical Biology | A4103 Van Vranken4.9 & Weiss | 978-0-8153-4214-4 Answer © www.garlandscience.com design by www.blink.biz There are two possible hybrid pairs: the DNA-binding domain–MS2 and the activation domain–iron regulatory protein (as shown) or DNA-binding domain–iron regulatory protein and activation domain–MS2 (not shown). RNA library GAL4 DNA-binding domains MS2 viral coat protein iron regulatory protein Introduction to Bioorganic Chemistry and Chemical Biology | A4104 Van Vranken & Weiss | 978-0-8153-4214-4 transcription 3 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.10 . OH O H O. O . N H N H . O O. O . N H N H O H N . O O H H N N H H . N H H O H N O . O OH O N H H N O Answer 4.11 The two lone pairs around the sulfur of the thioether are diastereotopic, as shown in the following structure. The enzyme is selective for one of these lone pairs, generating the diastereomer of SAM with a configurationally stable S configuration at sulfur. The reaction with methyl iodide reacts with both lone pairs at approximately equal rates, leading to a mixture of R and S sulfonium ions. The natural S diastereomer of SAM is a good substrate for methyltransferases, whereas the R diastereomer is not. NH2 N N N (S)-configuration +H N to Bioorganic Chemistry and +H | N N Chemical Biology Introduction 3 3 A4105 O Van Vranken & Weiss | 978-0-8153-4214-4 design by www.blink.biz -O C -O C © www.garlandscience.com S HO 2 : OH NH2 SAM 2 O S H3C HO N N N OH : N : 4 Introduction to Bioorganic Chemistry and Chemical Biology | A4106 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.12 design by www.blink.biz © www.garlandscience.com lariat O O B Ade ..O n O P -O O O O O P OO this P–O bond is P -O O cleaved by O O exposure to base Introduction to Bioorganic Chemistry and Chemical Biology | A4107 Van Vranken & Weiss | 978-0-8153-4214-4 The phosphate indicated with the arrow still has an adjacent © www.garlandscience.com design by www.blink.biz 2ʹ-OH group, whereas the other two phosphate diesters lack the adjacent 2ʹ-OH group. This 2ʹ-OH group can play a key role in mediating RNA cleavage, as discussed earlier in the chapter. Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.13 5'-GATCCGCACCGAGTTCAAGAACACCTTTTCAAGAGAGGTGTTCTTGAACTCGGTGTTCTTTTTTTCTAGAG-3' transcription sense U UU A A GUGGCUCAAGUUCUUGUGG 3'-GAGAUCUUUUUUUCUU AG G A antisense 5'-GAUCCG CACCGAGUUCAAGAACACCU Answer 4.14 N H N G2447 N H Introduction to Bioorganic Chemistry and Chemical Biology | A4108 N H& Weiss N O | 978-0-8153-4214-4 Van Vranken N N N design by www.blink.biz © www.garlandscience.com 1 1 N 3 ..N N H O N H H O P O O A2450 N A2451 3 : ..N H O N H N N H N A2451 N G2447 N O H O P O O A2450 Introduction to Bioorganic Chemistry and Chemical Biology | A4109 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.15 © www.garlandscience.com design by www.blink.biz Cyt Watson–Crick base pair H Ura N O N H N H N N H H O H N R N Gua N N O H N O H N H N N ManQ wobble base pair O H N R N Introduction to Bioorganic Chemistry and Chemical Biology | A4110 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.16 www.garlandscience.com design by www.blink.biz © ATG GGC CGC CGC CCC GCC CGT TGT TAC CGG TAT TGT AAG AAC AUG GGC CGC CGC CCC GCC CGU UGU UAC CGG UAU UGU AAG AAC Met Gly Arg Arg Pro Ala Arg Cys Tyr Arg Tyr Cys Lys Asn (The three-letter abbreviations used here correspond to the first three letters of the amino acid, with the exception of asparagine and glutamine, which are abbreviated Asn and Gln to avoid confusion with aspartic acid and glutamic acid, respectively.) Answer 4.17 A A A G G C 5'-G CA GCT G CT G CT CCA G TT-3' Ala Thr Asp Gly Ala Ala or or or or or or Glu Ala Ala Ala Pro Val Introduction to Bioorganic Chemistry and Chemical Biology | A4111 Van the Vranken & Weiss of | 978-0-8153-4214-4 So number possiple hexapeptides coded by these sequences is 26 = 64. © www.garlandscience.com design by www.blink.biz 5 Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.18 The Zn2+ ion acts a Lewis acid, coordinating to both the formamide carbonyl and the hydroxide nucleophile. The Zn2+ ion activates the carbonyl group toward attack and brings the hydroxide nucleophile into proximity with the carbonyl carbon. peptide deformylase active site Zn O peptide deformylase active site Zn actinoin + O - H N N H O HO ~ ~ N HO: H O + O hydroxide anion S protein/peptide O O H N N N H O R NH Introduction to Bioorganic Chemistry and Chemical Biology | A4112 Van Vranken & Weiss | 978-0-8153-4214-4 Answer 4.19 design by www.blink.biz © www.garlandscience.com A oxocarbenium ion HO HO OH HO OH O HO : H2NOC O: O+ : N + : 6 Me3 NAD+ N H H2NOC O O N+ Me3N+ N N+ N H H2NOC N NH H O O N NH H EF-2 Introduction to Bioorganic Chemistry and Chemical Biology | A4113 Van Vranken & Weiss | 978-0-8153-4214-4 B The enzyme lowers the entropic barrier by bringing design by www.blink.biz © www.garlandscience.com the reactive carbon of NADH into close proximity with the diphthamide residue. Carbocation formation is the rate-determining step in an SN1 reaction. The enzyme probably lowers the enthalpic barrier to ionization by stabilizing the oxocarbenium ion. Answer 4.20 Because chloramphenicol disrupts all protein translation, chloramphenicol will prevent the cells from expressing the plasmid-encoded protein (chloramphenicol acetyl transferase) that confers resistance. Allowing the cells to grow before exposure to chloramphenicol ensures an adequate supply of the proteins that confer resistance. Answer 4.21 Number of 50-mer RNAs = 450 = 1.3 × 1030 Molecular mass of a 50-mer DNA = 50 × 305 g mol–1 = 15,250 g mol–1 500 × 10–6 g × 1 mol/15,250 g × 6.02 × 1023 molecules mol–1 = 1.9 × 1017 1.9 × 1017/1.3 × 1030 = 1.6 × 10–13 (!) *Answer 4.22 A 3ʹ-CATAGCTGTCCTCCT-5ʹ 5ʹ-GUAUCGACAGGAGGA-3ʹ B 3ʹ-CATGGTGGT-5ʹ 5ʹ-GUACCACCA-3ʹ Answer 4.23 B *A 5' 3' 5' 3' UA A G G C C G G C C G GA CU GU G C CU CA C UG AG CC A G G G U U C U G C A C G G C C G C UG C G C G UG G G A C GA C CG Introduction to Bioorganic Chemistry and Chemical Biology: Answers to Chapter 4 Answer 4.24 *A O ONa P O O *B O ONa P O C O H2O 100 °C O P ONa + HO ONa 1 M NaOH OH O 1 M NaOH OH H2O 100 °C + O NaO P ONa OH O Ura OH *Answer 4.25 O (excess) ONa 1 M NaOH AdeB O CCG A CCG G OH G A O G G A NaO P O A A H2OA P OA A O G GNaO G G 100 U °C U O HO G G OH A A C C G C G C Introduction Chemical Biology U | A4115 U AG ribozyme to Bioorganic AG Chemistry andribozyme A C A C Van Vranken & Weiss | 978-0-8153-4214-4 5'-GCGCAGC ACGGC-3' 5'-GGCGACC ACCGU-3' design by www.blink.biz 3'-CGCGUCGCUGCCG-5' © www.garlandscience.com 3'-CCGCUGGCUGGCA-5' NaO P O O A substrate Ura O HO + OH ONa NaO P O O substrate Introduction to Bioorganic Chemistry and Chemical Biology | A4116 Van Vranken & Weiss | 978-0-8153-4214-4 www.garlandscience.com design by www.blink.biz ©*Answer 4.28 Note that the presence of start and stop codons confirms that the reading frame is correct. ACCESSION X69154; Glycine max ENOD40-1 mRNA. 1 61 121 181 241 301 361 421 481 541 601 661 gaauuccgcu GCUCACAACC gugcucacuc cugaucaaca cuccgacgga uggacuccau uguaauaaua uuacucaauu uaggcaauuu uguuuguguu ucaguuuagg aaaaaaaaaa aaaccaaucu AUCCAUGGUU cucacacucc agggaugugu ggagaggcuu uggggucucu aacaaaguug ugcagcugac gugaucacuc guuaguuaug auuguauucu aaaaaaaaa aucaaguccu CUUGAagaag cucacuuaaa ucuaacauuc ggcuacagcc auggcuaugu gucuuccuuu uagaauuccu ccuucccuuu accuuaugag auugaacuuu gauuaaucug cuuggagaga acaguuuguu uuucuugagu uggcaaaccg agugcucaug ugagaaguua uucucucuuc ucaugucuuc gaaauaaaag auuagaaaag gugagcAUGG aaggggugug uuggcuuagc ggcggaagca gcaagucaca uaguucuucu ccagcuuuug aguuucugca uguguucccu aauaguacaa uuuccagagu AGCUUUGUUG agaggagagg uuuggcuucu gauacacauu aaaaaggcaa ugcuguagaa cuguccaaaa gaugaguagg uuuccaugcu uucuaguccc ccuuucuaaa Peptide A (highlighted) AUGGAGCUUUGUUGGCUCACAACCAUCCAUGGUUCUUGA MetGluLeuCysTrpLeuThrThrIleHisGlySerSTOP Peptide B (underlined) AUGGUUCUUGAagaagcuuggagagaaaggggugugagaggagagggugcucacuccucacacucccucacuuaa MetValLeuGluGluAlaTrpArgGluArgGlyValArgGlyGluGlyAlaHisSerSerHisSerLeuThrSTOP Introduction to Bioorganic Chemistry and Chemical Biology | A4118 Van Vranken & Weiss | 978-0-8153-4214-4 *Answer 4.31 © www.garlandscience.com design by www.blink.biz Most amine-containing buffers are tertiary trialkylamines that can form stable adducts through acylation or sulfonylation. Tris is a primaryl alkylamine that can react readily with DEPC to form stable carbamates. HO HO Tris .. NH2 OH O + EtO pH 8 O O OEt HO HO O N H OH Introduction to Bioorganic Chemistry and Chemical Biology | A4121 Van Vranken & Weiss | 978-0-8153-4214-4 © www.garlandscience.com design by www.blink.biz OEt O HO Ade OH 7