Intermediate I year(2013) CHEMISTRY QUESTION BANK IPE
advertisement

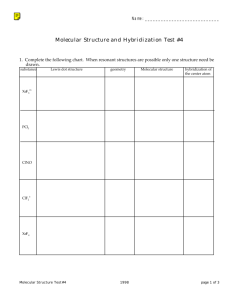
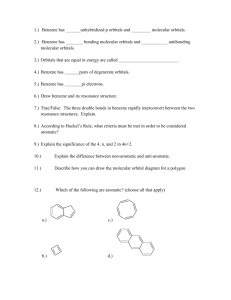
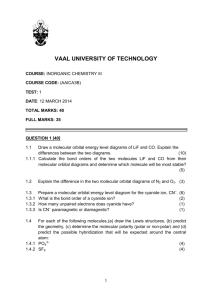
Intermediate I year(2013) CHEMISTRY QUESTION BANK IPE Exams LONG ANSWER QUESTIONS (8 marks) 1. What are quantum numbers? Explain the significance of various quantum numbers. 2. Write any three methods of preparation of Ethane. Also give its three chemical properties. 3. A) Describe any two methods of preparation of ethylene. Give equation for the reaction of ethylene with the following: a) Ozone b) Hypohalous acid c) cold and dil. Alk KMnO 4 d) Heated with O2 at high pressure. 4. What is periodic property? How do following properties change in a group and period? a) Atomic radius b) Ionization potential c)Elecronegetivity 5. Discuss hybridization with suitable examples. 6. State Bohr’s atomic postulates. Explain the different lines in various series of hydrogen spectrum. 7. Write an essay on VSEPR theory. 8. Describe the two methods of preparation of benzene. Explain the Halogenation, Nitration, Sulphonation and Alkylation of benzene. 9. Define atomic orbital. Explain the shapes of s, p and d orbitals with the help of diagrams. 10. Explain i) Aufbau Principle ii) Hund’s Rule iii) Pauli’s Principle iv) De-broglie’s hypothesis 11. Define IE1 and IE2. Why is IE2>IE1 for a given atom? Discuss the factors that affect IE of the element? 12. Write an essay on s, p, d and f block elements. 13. Given the molecular orbital energy diagram of a) N2 and b) O2. Calculate the respective bond order and discuss the magnetic nature. 14. Give two methods of preparation of acetylene. How does it react with water and Ozone? 15. Describe any two methods of preparation of benzene? Explain the halogenation, Alkylation, acylation, nitration and sulphonation of benzene. Short Answer questions (4 marks) 1. What is perhydrol? Give structure of Hydrogen Peroxide. 2. Balance the equations in acid medium by ion electron method: Br- + SO42Cr2O72- + NO2 - Br2 + SO2 Cr3+ + NO3- 3. What is oxidation no. of Mn in a) KMnO4 b) MnSO4? 4. Describe various methods of preparation of hydrogen peroxide. 5. Draw MOED of N2 and O2. 6. Explain the structure of XeF2 and XeF4. 7. Describe the preparation of NaOH by Nelson Cell. 8. 100 cm3 of CO2 gas is diffused in 25 seconds through a porous membrane. How much time does the same volume of SO2 gas to diffuse? 9. What is hard water? How is hardness of water removed by i) permutite method ii) Calgon method. 10. Discuss the significance of De-Broglie’s hypothesis. 11. Define IP1 and IP2.Why IP2 is greater than IP1? 12. State and explain Dalton’s Law of partial pressures. 13. Explain Markownikov rule with a suitable examples. 14. State and explain a) Pauli’s exclusion principle b) Hund’s rule. 15. Discuss the conformations of ethane. 16. What is ozonolysis? Which types of compounds react with ozone? Explain with an example. 17. Discuss factors effecting IP values of elements. 18. Write about Dewar’s method for the separation of noble gases from the mixture. 19. Deduce Boyel’s Law, Charle’s law, Graham’s Law of diffusion from kinetic gas equation. 20. State the postulates of Kinetic molecular theory of gases. 21. Explain the structure of diborane. 22. Explain the structure of boric acid. 23. Explain borax bead test with suitable examples. 24. Explain sp3d hybridization with an example. 25. Explain Homologous Series with example. 26. Explain the hybridization involved in PCl5 molecule. 27. Explain the hybridization involved in SF6 molecule. 28. Define a) RMS b) average and c) most probable speed of gas molecules. Give their interrelationship and formulas. 29. Find the RMS, most probable and average speeds of SO2 at 270C. 30. Determination of Empirical formula and molecular formula-problems 31. State the first law of thermodynamics. Explain its mathematical notation. 32. State and explain Hess’s law of constant Heat summation. 33. Define heat capacity. What are Cp and Cv? Show that Cp-Cv=R. 34. What is Lechatlier’s principle? Discuss briefly the factors which can influence the equilibrium. 35. Explain the concept of Bronsted acids and Bronsted bases. Illustrate the answers with suitable examples. 36. Define pH. What is buffer solution? 37. What is solubility product? 38. What is common ion effect? 39. What do you know about Castner- Kellner process? Write the principle involved in it. 40. Give an account of biological importance of Na+ and K+ ions. 41. What is Plaster of Paris? Write a short note on it. 42. Discuss the various reactions that occur in Solvay process. 43. Explain the difference in properties of diamond and graphite on the basis of their structure. 44. Why is diamond hard? 45. What are silicones? How are they prepared? Give one example. What are their uses? 46. Write a note on a) Silicates b) Zeolites c) Fullerenes 47. Explain the structure of silica. How does it react with a) NaOH b) HF 48. What is substitution reaction? Explain any two substitution reactions of benzene. 49. What do you understand about geometrical isomerism? 50. Explain the method of writing E-Z configurations for geometrical isomers taking CHCl=CFBr as your example. 51. Discuss Markownikov’s rule and Kharash effect. VERY SHORT ANSWER QUESTIONS (2 marks) 1. How many moles of glucose are present in 540 grams of glucose? 2. What is biological importance of Mg2+ and Ca2+ ions? 3. Define Heisenberg’s uncertainty Principle. 4. State Dalton’s Law of Partial pressures. 5. Calculate the equivalent weight of sodium carbonate. 6. Give electronic configuration of Cu and Cr atoms. 7. What is position isomerism? Give example 8. Give two uses of heavy water. 9. A carbon compound has empirical formula CH2O. Its molecular weight is 90.Find its molecular formula. 10. Write the structures of following compounds : a) 3-Chloro-4-Methyl hexane. b) 2-Methoxy-3,3-dibromo-1-pentanol 11. Give equations for the formation of caustic soda from NaCl. 12. Give an example for Wurtz reaction. 13. What are coordination numbers of NaCl and CsCl crystals? 14. What is the kinetic energy of 5 moles of N2 gas at 270C in calories? 15. What is functional group isomerism? Give an example. 16. What is Boltzmann constant? Give its value. 17. What is Metamerism? Give example. 18. Write four differences between sigma and pi bond. 19. State Graham’s Law of Diffusion. 20. What is COD, BOD and TLV? 21. Name any four green house gases. 22. What are the causes of acid rains? 23. What are CFC’s? What is the harm of them? 24. Graphite is a good conductor. Explain. 25. Define Receptor, Sink and Speciation. 26. Explain Nalgonda deflouridation technique. 27. What is Eutrophication and Bioamplification? 28. Write the products formed in following reaction CH2=CH2 + O 3 A Zn/H2O B 29. What is Diagonal relationship? Give an example. 30. What is Hund’s rule? 31. Explain the term “SYNGAS”?