Gummy Bear Lab
advertisement

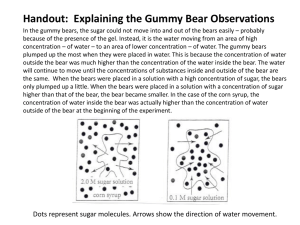
Name:_________________________________________ Period:________ Partner :__________ Gummy Bear Osmosis Lab (CP Bio) 1. Introduction: (Read this and underline the key words!) Molecules are in constant motion, and tend to move from areas of higher concentrations to lesser concentrations. Diffusion is defined as the movement of molecules from an area of high concentration to an area of low concentration. The diffusion of water molecules through a selectively permeable membrane is known as OSMOSIS. Selectively permeable means that some molecules can move through the membrane while others cannot. Movement through membranes is called transport. Diffusion and osmosis are passive forms of transport; this means that do not need energy to move areas of high concentration to areas of low concentration. Active transport requires energy to transport molecules from low concentration to high concentration. Gummy Bears are popular candies made of gelatin, starch, and sugar. 2. Purpose: To measure/observe the process of osmosis by soaking a gummy bear in various solutions. 3. Pre-Lab Question: How will soaking Gummy Bear candies in distilled water affect the size of the candy? ____________________________________________________________________________________________________ 5. Hypothesis (If, Then Statement): You need a hypothesis for EACH DAY. ___________________________________________________________________________________________________ 6. Materials: Beakers Rulers 7. Procedure: Wax Paper Distilled Water Paper Towels Gummy Bears Scale (mass) Calculator Day 1 1. Use the masking tape to label your beaker with your names & class period. 2. Use the ruler to find the measurements of your bear. (See below) 3. Use a scale to find the mass (g) of your candy bear. a. Use a piece of wax paper to protect the pan of the balance. b. Remember to subtract the mass of the wax paper (or zero it). 4. Record observations/data about the candy bear. (see table below) 5. Fill your beaker ½ way full with distilled water. (_______mL) 6. Put your candy bear in the water; record observations. 7. Set the beaker aside for one day. Day 2: 8. After the candy bear has been in the distilled water overnight, gently take it out of the water and pat it dry. Be very careful because the candy is now extremely breakable. 9. Repeat steps 2 – 4 and record this under your day 2 category in the data table. END OF DAY 1 EXPERIMENT Day 2: Design your own experiment: In a group of 4 you are to design your own method for how to shrink your gummi bear back to normal size. Write your own procedure step by step and carry out the procedure in your lab. After your complete the lab write all DATA in your lab notebooks AND on this data table. ANSWER THE QUESTIONS AT THE END OF THIS LAB AS YOU FINISH EACH DAY’S WORK. 8. Data Analysis Tables/ Calculations Data: Data Table 1: Gummi Bear Measurements/ Observations Bear Color Day Height Width Thickness Surface Area Mass Volume Density 1 2 Change 3 Change 1. Calculate the volume of the gummi bear using a graduated cylinder. a. Initial volume reading of the graduate: _____________cm3. b. Volume reading after you add the gummi bear: __________ cm3. c. Volume of the gummi bear (b – a) ______________________ cm3. 2. Calculate the surface area of the gummi bear. Show all work. (H - height; W – width; T – thickness) 2(HW) + 2(WT) + 2(HT) = 3. Calculate the density of the gummi bear. Show all work. Mass ÷ Volume = 4. Calculate the concentration of sugar in your gummi bear. (Each bear has 1.8 g of sugar in it.) Mass of sugar ÷ volume of the bear = 5. Place your gummi bear in a beaker and draw your set up. Color it! DAY 2: 1. What happened to your gummi bear? Which did will the water move? Draw a picture. Inside the bear Concentration of sugar: _________ Outside the bear Concentration of sugar: _________ _________ sugar. _________ sugar. _________ water. _________ water. 2. Is the solution you put your gummi bear in yesterday hypertonic or hypotonic? Explain. 3. Calculate the volume of the gummi bear using a graduated cylinder. a. Initial volume reading of the graduate: _____________cm3. b. Volume reading after you add the gummi bear: __________ cm3. c. Volume of the gummi bear (b – a) ______________________ cm3. 4. Calculate the surface area of the gummi bear. Show all work. (H - height; W – width; T – thickness) 2(HW) + 2(WT) + 2(HT) = 5. Calculate the density of the gummi bear. Show all work. Mass ÷ Volume = 6. Calculate the concentration of sugar in your gummi bear. (Each bear has 1.2 g of sugar in it.) Mass of sugar ÷ volume of the bear = DAY 3: (Your OWN Experimental Design Results!) 1. Calculate the volume of the gummi bear using a graduated cylinder. a. Initial volume reading of the graduate: _____________cm3. b. Volume reading after you adds the gummi bear: __________ cm3. c. Volume of the gummi bear (b – a) ______________________ cm3. 2. Calculate the surface area of the gummi bear. Show all work. (H - height; W – width; T – thickness) 2(HW) + 2(WT) + 2(HT) = 3. Calculate the density of the gummi bear. Show all work. Mass ÷ Volume = Analysis Questions: 1. 2. 3. 4. 5. 6. 7. 8. Compare your gummi bear from how it looked/measured on Day 1 to Day 2. Why did these changes occur; what is happening? How do you know the gummi bear was in a hypotonic solution? Did you notice a color change in your water on Day 2? Why? For your own experimental set up; describe what you are doing and why you think it will work. Draw a picture of your set up below. Did your experimental design work; why or why not? What could you have done differently? Write a conclusion for the lab. This should be at least 1 FULL page typed, single spaced and using citations.