Chemistry 161
advertisement

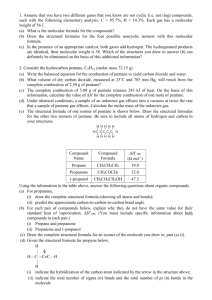
Chemistry 161 Lecture 1: Types of Matter – Macroscopic and Atomic Views Sections 1.1, 1.2 in Gilbert, Kirss, Foster, and Davies 2nd edition 09/25/08 HW #1: 1.6, 1.10, 1.14, 1.20, 1.28, 1.38, 1.48, 1.62, 1.64, 1.84 Learning Objectives: 1. Define the basic terms associated with matter 2. Identify the principal elements 3. Introduce the means of representing chemical formulas 1.1 & 1.2: What’s the Matter? Matter: Has mass and takes up space Chemistry: The fundamental study of matter. By fundamental, we refer to the modern view of chemistry based on the building blocks found in the periodic table (from the Greek atomos, meaning “not to cut”). The idea of the indivisible unit was put forward by Democritus, ca. 460 – 370 BC The Classification of Matter Please refer to figure 1.1 Compound is a “catch all” for any pure non-elemental substance. Properly, the term molecule is reserved for covalently bonded species o Covalent species are ones which share electrons to achieve a noble gas electronic configuration – much more on this is critical concept to come The importance of homogeneity in terms of concentration and delivering accurate quantities: 1 Familiar groups and elements you will want to know: Important Elements Based on Group Behavior/Position in the Periodic Table o Bear in mind that the elemental forms are not necessarily found in nature; in fact, with the exception of a few native metals (Au being one of them); the nonmetals N2, O2 & S; and the noble gases, elemental forms are quite rare o Alkali Metals: o Alkaline Earth Metals: o Group 16: o Halogens: o Noble Gases: o Periods 1, 2, and 3: 2 On Depicting Chemical Compounds Chemical Formula Iron(III) oxide (rust) Magnesium sulfate heptahydrate (epsom salts) Acetic acid (main component in vinegar) Pentane (first liquid saturated hydrocarbon) Structural formula Iron(III) oxide (rust) Magnesium sulfate heptahydrate (epsom salts) Acetic acid (main component in vinegar) Pentane (first liquid saturated hydrocarbon) Condensed Structural Formula Acetic acid (main component in vinegar) Pentane (first liquid saturated hydrocarbon) Line-angle formula Acetic acid (main component in vinegar) Pentane (first liquid saturated hydrocarbon) 3 Chemistry 161 Lecture 2: Separation of Mixtures, Properties & States of Matter Sections 1.1, 1.2 in Gilbert, Kirss, Foster, and Davies 2nd edition 09/26/08 Learning Objectives: 1. Define mixtures and introduce means of separating them 2. Differentiate between physical properties, chemical properties, physical changes, and chemical changes 3. Define intensive vs. extensive properties 4. Describe density as an intensive physical property 5. Describe density as a useful conversion factor to convert between mass and volume 6. Examine 2 common methods for determining density Differential Solubility and Extraction or Filtration 4 Distillation Chromatography – requires a mobile phase and stationary phase o Paper o Thin layer o Column o Gas o Liquid Question: What physical property is being used to separate mixtures in chromatography? 5 Generally, separating mixtures involves physical changes since the compounds making up the mixture are not transformed; however, there are occasions where difficult separations may require reaction before differences in physical properties are exploited Sometimes we start with pure compounds and create mixtures, where the physical properties of the properties of the mixture are greatly preferred to any of the components Lotions are mixtures (oil in water emulsions) as are creams (water in oil emulsion) In 1937, 107 people, mostly children, died when given sulfanilamide containing1 diethylene glycol. This incident gave rise to the Food, Drug, and Cosmetic Act of 1938, which essentially gives the FDA its statutory authority. Here the mixture was not such a good thing o Amphipathic defined: Chemical properties: the tendency of a given atom or molecule to react under a given set of conditions as well as the characteristics of the transformation Physical properties: everything else – color, odor, melting point, boiling point, density, hardness, electrical conductivity, phase under a given set of pressure and temperature conditions, etc. Chemical changes: Chemical reactions, where the bonding between atoms within a molecule changes Physical changes: Conversion of atoms or molecules from one phase to another; generation or purification of mixtures 1 The diethylene glycol was intentionally added by the manufacturer, the S.E. Massingill Co.,to help dissolve the sulfanilamide and sweeten the taste 6 A final word on the notion of purity… Are any substances truly pure? From a practical we establish purity limits as an example, the legal limit for As contamination is 10 PPB, which is equal to 10 ng/mL or 1 mg/100 L Density and Dimensional Analysis Density is defined as d = mass/V Density is an intensive physical property and an important practical property of compounds o Reference texts (CRC Handbook of Chemistry & Physics, Merck Index, etc) will give values for density, particularly for liquid compounds. Symbols typically used are d or , with a superscript indicating temperature o It is not unusual to see a subscript of 4 when density is listed (e.g. d 420 = .789). What is being reported is specific gravity and is a ratio of the density of the compound in question (at the temperature indicated) to water at 4 oC. Note that specific gravity has no associated unit and is independent of the forces of gravity Density may be used to convert mass to volume or volume to mass; in a sense it acts as a conversion factor Question: What is the mass of a liquid that has a volume of 18 mL and has a density of 1.0 g/mL? 7 Question: What is the volume occupied by 18.4 g of concentrated sulfuric acid, which has density = 1.84 g/mL? Question: How might you calibrate a 1 mL air displacement pipette, knowing that water has a density of .997 g/mL at room temperature? For regularly shaped (easily mathematically defined) objects, density may be determined by measuring mass and calculating volume directly. For irregularly shaped objects, the “Eureka” method is best used. Question: 00 Buckshot has a nominal diameter of .180” and has a nominal mass of .567 g for lead shot. What is the density of 00 Buckshot? Question: What is the density of a gold crown that displaces 102.3 mL and has a mass of 1974 g? 8