REVIEW MODULE 6 LEARNING GOAL 2d.) Investigate the effects of
advertisement

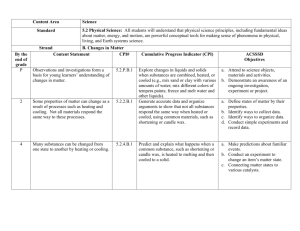
REVIEW MODULE 6 LEARNING GOAL 2d.) Investigate the effects of chemical change on physical properties of substances (e.g. rust, fire, respiration, photosynthesis). VOCABULARY: 1.) chemical change: A synonym for chemical reaction. A change that rearranges atoms into new molecules and produces new substances with new properties. 2.) Products: The substances that are produced due to a chemical change. Another word for new substance. EXAMPLE: white powder, 3.) Reactants: The substances that enter into a chemical change. The starting substances. LEARNING ACTIVITIES Chemical Changes Change Everything Demos Chemical Changes Change Everything Demos-Key ESSENTIAL QUESTIONS S1O2d- 1.) Explain why chemical changes change BOTH chemical and physical properties. Use the the words reactants and products. S1O2d-2.) Explain at least one example of how a chemical change changed both physical properties and chemical properties from your demos. Describe the properties before AND after. FORMATIVE QUIZ 6a 1. What bests describes what happens to physical properties during a chemical change? a. New substances are created and have the same physical properties as the reactants b. New substances are not created and have the same physical properties as the reactants c. New substances are not created and have the same physical properties as the reactants d. New substances are created and have different physical properties as the reactants 2. The substances that are produced due to a chemical change. Another word for new substance. a.Oxidation b. products c. Rusting d. reactants 3. The substances that enter into a chemical change. The starting substances. a. Oxidation b. Rusting c. products d. reactants. 4. A synonym for chemical reaction. A change that rearranges atoms into new molecules and produces new substances with new properties. a. Chemical Change c. products b. Physical Change d. reactants. 5. What is true of physical changes? a. New substances are formed with new chemical and physical properties. b. The same substances are present with the same chemical properties andsome different physical properties. c. The same substances are present with new chemical properties and some different physical properties and physical properties. d. New substances are formed with different chemical and physical properties. 6. What is true of chemical changes? a. New substances are formed with different chemical and physical properties. b. They change only physical properties c. They change neither chemical properties not physical properties d. They change only chemical properties 7.) Use the table below to help answer the following question. An experiment was done by mixing together various substances. When these substances were mixed some of them went through a chemical change. Use the physical properties data below to determine which went through a chemical change. Which combinations of substances probably went through a chemical change? a. Substance 1 and Substance 2 b. Substance 4 and substance 3 c. Substance 5 and Substance 3 d. Substance 5 and Substance 4 Reactants Metallic Yellow liquid Malleable Products Metallic Yellow Liquid Malleable Liquid at room temperature Green and clear Substance 6 and substance 4 Solid at room temperature Metallic yellow Brittle Liquid at room temperature Colorless liquid Substance 5 and Substance 3 Light blue powder Yellow liquid Substance 1 and Substance 2 Substance 4 and substance 3 Solid at room temperature Blue with small metallic pieces Light blue powder Yellow liquid Substance 5 and Substance 4 Substance 6 and Substance 4 Light blue powder Colorless liquid Liquid at room temperature Colorless liquid Light blue powder Colorless liquid Liquid at room temperature Colorless liquid ESSENTIAL QUESTIONS KEY S1O2d- 1.) Explain why chemical changes change BOTH chemical and physical properties. Use the the words reactants and products. During a chemical change the reactants change into completely different substances. These new substances are called products and they have completely different properties because they are new substances. S1O2d-2.) Explain at least one example of how a chemical change changed both physical properties and chemical properties from your demos. Describe the properties before AND after. When we burned the sugar it was a chemical change. Combustion occurred and created a brand new substance. The sugar ahd a physical property of being grainy and white. The product was black and gooey. These are physical properties and they were different before and after the chemical change. Also, the sugar had the chemical property of being combustable, but the new black substance was not combustible. FORMATIVE QUIZ 6 KEY 1. What bests describes what happens to physical properties during a chemical change? a. New substances are created and have the same physical properties as the reactants b. New substances are not created and have the same physical properties as the reactants c. New substances are not created and have the same physical properties as the reactants d. New substances are created and have different physical properties as the reactants 2. The substances that are produced due to a chemical change. Another word for new substance. a.Oxidation b. products c. Rusting d. reactants 3. The substances that enter into a chemical change. The starting substances. a. Oxidation b. Rusting c. products d. reactants. 4. A synonym for chemical reaction. A change that rearranges atoms into new molecules and produces new substances with new properties. a. Chemical Change c. products b. Physical Change d. reactants. 5. What is true of physical changes? a. New substances are formed with new chemical and physical properties. b. The same substances are present with the same chemical and physical properties. c. The same substances are present with new chemical and physical properties. d. New substances are formed with different chemical and physical properties. 6. What is true of chemical changes? a. New substances are formed with different chemical and physical properties. b. They change only physical properties c. They change neither chemical properties not physical properties d. They change only chemical properties 7.) Use the table below to help answer the following question. An experiment was done by mixing together various substances. When these substances were mixed some of them went through a chemical change. Use the physical properties data below to determine which went through a chemical change. Which combinations of substances probably went through a chemical change? a. Substance 1 and Substance 2 b. Substance 4 and substance 3 c. Substance 5 and Substance 3 d. Substance 6 and Substance 4 Reactants Metallic Yellow liquid Malleable Products Metallic Yellow Liquid Malleable Liquid at room temperature Green and clear Substance 6 and substance 4 Solid at room temperature Metallic yellow Brittle Liquid at room temperature Colorless liquid Substance 5 and Substance 3 Substance 5 and Substance 4 Substance 6 and Substance 4 Light blue powder Yellow liquid Light blue powder Colorless liquid Liquid at room temperature Colorless liquid Substance 1 and Substance 2 Substance 4 and substance 3 Solid at room temperature Blue with small metallic pieces Light blue powder Yellow liquid Light blue powder Colorless liquid Liquid at room temperature Colorless liquid