Soap Making in Fight Club_20Mar2010
advertisement

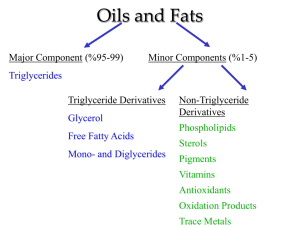
Saponification One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye potash (potassium hydroxide). Hydrolysis of the fats and oils occurs, yielding glycerol and crude soap. In the industrial manufacture of soap, tallow (fat from animals such as cattle and sheep) or vegetable fat is heated with sodium hydroxide. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. The water layer is drawn off the top of the mixture and the glycerol is recovered using vacuum distillation. The crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide, and glycerol. These impurities are removed by boiling the crude soap curds in water and re-precipitating the soap with salt. After the purification process is repeated several times, the soap may be used as an inexpensive industrial cleanser. Sand or pumice may be added to produce a scouring soap. Other treatments may result in laundry, cosmetic, liquid, and other soaps. Dynamite Nitroglycerin is a heavy, colorless, oily, explosive liquid obtained by nitrating glycerol. Nitroglycerin was the first adopted as a commercially useful explosive by Alfred Nobel. He experimented with safer ways to handle the dangerous substance; his younger brother Emil and several workers were killed in 1864 in a nitroglycerin explosion at the family's armaments factory in Heleneborg, in Sweden. Alfred Nobel developed the use of nitroglycerin as a blasting explosive by mixing the nitroglycerine with inert absorbents (known as diatomite or kieselgur, a naturally occurring, soft, siliceous sedimentary rock that is easily crumbled into a fine white to off-white powder). He named this explosive dynamite and patented it in 1867. Nitroglycerin is a high explosive that is so unstable, at the slightest jolt, friction or impact can cause it to detonate. The molecule contains oxygen, nitrogen, and carbon so when it explodes a lot of energy is released as the atoms rearrange to form new molecules with strong, stable bonds like N2 and CO. However, it is the speed of the decompostition reaction which makes it such a violent explosive. A supersonic wave passing through the material is what causes the high explosive material to decompose almost instantaneously. This instantaneous destruction of all molecules is called a detonation, and what causes the destructive blast is a result from the rapid expansion of hot gases. Nitroglycerin has an advantage over some other high explosives, that no visible smoke is produced, therefore being able to be a "smokeless powder".[