160-B - St.Joseph's College
advertisement
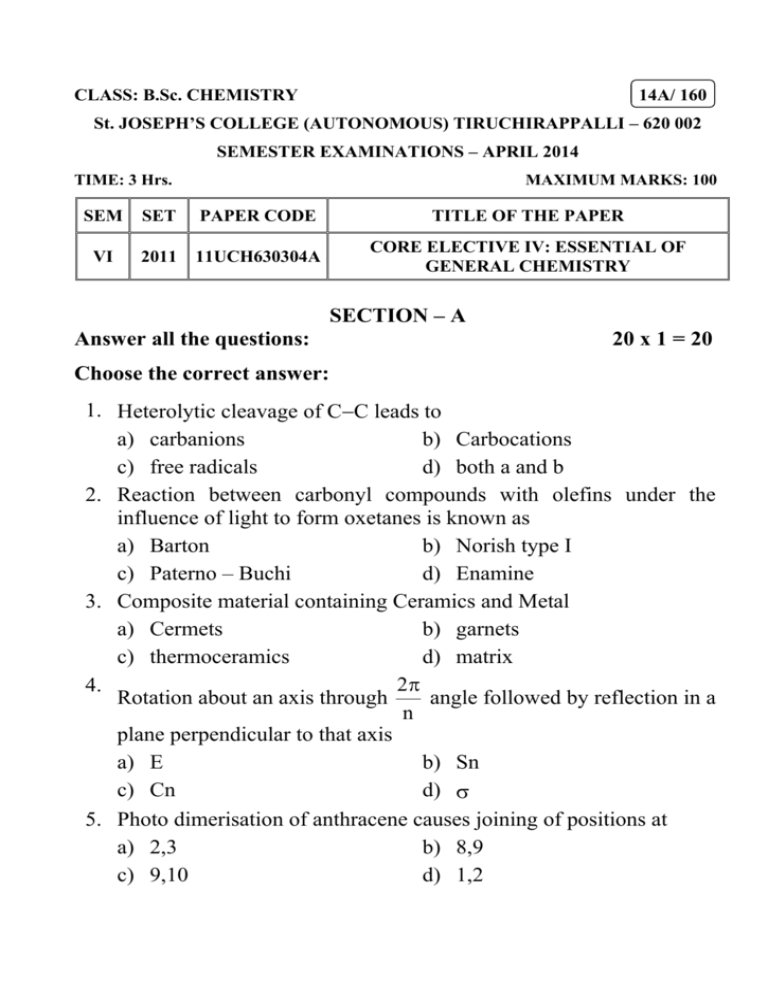
CLASS: B.Sc. CHEMISTRY 14A/ 160 St. JOSEPH’S COLLEGE (AUTONOMOUS) TIRUCHIRAPPALLI – 620 002 SEMESTER EXAMINATIONS – APRIL 2014 TIME: 3 Hrs. MAXIMUM MARKS: 100 SEM SET PAPER CODE TITLE OF THE PAPER VI 2011 11UCH630304A CORE ELECTIVE IV: ESSENTIAL OF GENERAL CHEMISTRY SECTION – A Answer all the questions: 20 x 1 = 20 Choose the correct answer: 1. Heterolytic cleavage of CC leads to a) carbanions b) Carbocations c) free radicals d) both a and b 2. Reaction between carbonyl compounds with olefins under the influence of light to form oxetanes is known as a) Barton b) Norish type I c) Paterno – Buchi d) Enamine 3. Composite material containing Ceramics and Metal a) Cermets b) garnets c) thermoceramics d) matrix 2 4. Rotation about an axis through angle followed by reflection in a n plane perpendicular to that axis a) E b) Sn c) Cn d) 5. Photo dimerisation of anthracene causes joining of positions at a) 2,3 b) 8,9 c) 9,10 d) 1,2 Fill in the blanks: 6. Neighbouring group participation always resulted in _______ of configuration. 7. Cleavage of Cl2, 00 linkages in peroxide are the examples for _______ reaction. 8. Laminates and sandwich panels are examples for _______ composites. 9. Inversion through a point is termed as _______. 10. Percentage of water used in Fricke dosimeter is _______. State True or False: 11. Ozonolysis of terminal alkenes always give methanol as one of the products. 12. Synthons are structural units within a molecule which can be formed by known or conceivable synthetic operation. 13. Reinforced composites are prepared by calcination process. 14. The point group symmetry of the staggered CH3 – CH3 is C3V. 15. Nitric oxide helps the photochemical combination of H2 and Cl2. Match the following: 16. Change of polarity of carbonyl compound - a) Sensitizer 18. Fibre reinforced composites - b) Pultrusion c) Umpolung 19. E 20. Hg - d) Norrish type II e) identity 17. elimination of Carbonyl compound with -H SECTION – B Answer all the questions: 5 x 4 = 20 21. a. Write down the products obtained when 1,3-penta diene undergoes ozonolysis. OR b. Acetylene gives acetylides whereas dialkylderivative does not. Why? 22. a. Explain the mechanism of sigmatropic rearrangement. OR b. Explain the mechanism of claisen rearrangement. 23. a. How are composite materials classified? OR b. Explain any one method of preparing fibre reinforced composite. 24. a. List out the symmetry elements and point groups symmetry of S8, B2H6 and [Co(en)3]3+. OR b. Show the symmetry element operators form a group by C2V as an example. 25. a. Explain Chemi luminescence with examples. OR b. Write notes on radiolysis of water. SECTION – C Answer any FOUR questions: 4 x 15 = 60 26. a. Discuss the properties of aliphatic and aromatic carbonyl compounds. (10) b. Explain Unpolung with an example. (5) 27. a. Describe Jabalonski diagram. b. Distinguish reactions. photochemical (5) reactions from c. Explain Barton reaction. thermal (5) (5) 28. a. Discuss the components, types, products and methods of fabrication of Ceramics and wares. (12) b. Write notes on glazing. (3) 29. a. Explain the point group symmetry and elements present in benzene, chair & boat chclo hexane. (6) b. State and explain the great orthogonality theorem. (4) c. Describe the criteria for optical activity and dipolemoment on the basis of symmetry. (5) 30. a. Write notes on (i) H2Cl2 photochemical reaction (ii) Photosensitisations b. Explain the dosimetry of Fricke dosimeter. ************** (6) (4) (5)