Dr Lampman's Chem 354 Syllabus
advertisement

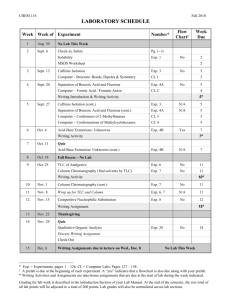
CHEMISTRY 354 Winter 2006 Dr. Gary Lampman Office Hours: CB 340, MTuWF 10 or at other times E-mail address: lampman@chem.wwu.edu Tuesday 3-6 PM CB 310 Thursday 3-6 PM CB 310 Telephone: 650-3151 Webpage for Chemistry 354: http://www.chem.wwu.edu/dept/facstaff/lampman/lamp354.html Required materials for the lab course: 1) Textbook: Pavia, Lampman, Kriz, and Engel, Introduction to Organic Laboratory Techniques, A Microscale Approach, Third Edition, Saunders College Publishing, 1999. 2) Chemistry 354 Prelabs and Lab Reports are available from my website, see above URL 3) Bound lab notebook (see page 5 of syllabus). 4) lab goggles (approved). Friday Lectures and Exams Laboratory lectures will be held in SL 120, on Fridays, at 2pm, for all of the laboratory sections. Dr. Lampman will be giving these lectures. The material covered will include theory and the techniques used in the experiments. In addition, he will lecture on each individual experiment in advance of when you will perform the experiment in the laboratory. The examinations will be designed to test your understanding of the theory and techniques of the experiments, as well as testing for safe laboratory practice. Each examination will be worth 100 points. There will be no make-up examinations offered. If you have an urgent and legitimate conflict, contact Dr. Lampman in advance! Sample exams will be available from my website. The two laboratory exams will be administered in SL 120 according to the following schedule: Midterm exam: Final exam (non-comprehensive): Friday, February 10th in SL 120 Tuesday, March 14th in SL120, 3:30 - 5:30 DATE TOPIC AND ASSIGNMENT January 6 Introduction to Microscale; Solubility READ: pages 2 - 49 Experiment 2 (pp. 49-60) Technique 1 (pp. 512-521) Technique 2 (pp. 521-528) Technique 3 (pp. 529-545) Technique 4 (pp. 545-558) January 13 Crystallization READ: Experiment 3 (pp. 60-71) Technique 5 (pp. 558-576) January 20 Chromatography Experiment 5 (pp. 80-87) Technique 6 (pp. 576-584) Technique 12 (pp. 669-692) Technique 14 (pp. 697-711) January 27 Distillation; Gas Chromatography READ: Experiment 6 (pp. 87-93) Technique 6 (pp. 585-595) Technique 8 (pp. 617-629) Technique 10 (pp. 644-662) Technique 15 (pp. 711-726) February 3 Caffeine from Tea Preparation of a C-4 or C-5 Acetate Ester. Topics for midterm test. READ: Experiment 11 (pp. 123-127) Experiment 52 (pp. 472-475) Essay “Caffeine” (pp. 119-123) Essay “Esters – Flavors and Fragrances” (128-130) Experiment 12 (pp. 131-135) Technique 7 (pp. 595-617) Technique 16 (pp. 726-731) Technique 19 (pp. 742-755) Appendix 3 (pp. A14-A32) February 10 MIDTERM EXAMINATION February 17 Nucleophilic Substitution READ: Experiment 19 (pp. 194-202) Lecture textbook, Chapter 10 Technique 15 (pp. 711-726) Technique 19 (pp. 755-760) Appendix 4 (pp. A32-A50) February 24 Aromatic Nitration READ: Experiment 40 (pp. 342-346) Experiment 54 (pp. 479-480) Technique 5 (pp. 558-576) Technique 19 (pp. 742-755) March 3 Procedures for Clean-Up and Check-Out. Topics for test. March 10 NO CLASS TODAY March 14 NOTE: This class meeting is on Tuesday FINAL EXAMINATION (3:30 to 5:30 PM) Organic Chemistry Laboratory Schedule January 5 The organic chemistry safety presentation on the web is available on the web at the following address: http://atom.chem.wwu.edu/dept/osafety/index.htm. Read pp 4 – 20, pp 521 – 528 and pp 529 - 530 in the lab book. We administer the safety quiz online at the following URL: http://argon.chem.wwu.edu/SafetyQ/OCQLink.htm A link to the safety quiz is included at the bottom of the instruction page. You will have 30 minutes to complete the 25 question quiz. First Try: Wednesday, January 4, 8AM to Friday, January 6, 5 PM (80% is passing; 20 correct) Second Try: Friday, January 6, 5:30 PM to Tuesday, January 10, 5 PM (84% is passing; 21 correct) Third Try: Tuesday, January 10, 5:30 PM to Thursday, January 12, NOON (88% is passing; 22 correct) In order to be enrolled in this course, each student will be required to take and pass a laboratory safety quiz. You must pass the quiz before Thursday, January 12th at NOON. If you cannot successfully pass the quiz, you will be dropped from this course. Lab: Check-in to lab and Introduction to Microscale, pp 38-49 Lab Exercise 1 (option A and B): measurement of volumes of liquids using an automatic pipet and a dispensing pump, pp 43 - 46, and Lab Exercise 2: calibrate Pasteur pipets, pp 46 and 47. No lab report for this exercise. January 10 Lab: Solubility lab (Exp 2, parts A-D only). Read Solubility lab (Exp 2, pp 49-59) and Technique 1 (pp 512 - 521) in advance of the laboratory. Prelab Report: turn in the Solubility prelab report. January 12 Lab: Start Crystallization lab (Exp 3, parts A, B and C, only). Read Crystallization lab (Exp 3, pp 60-71) and any technique references mentioned on page 63. Prelab Report: turn in the Crystallization prelab report. January 17 No lab today January 19 Lab: Finish Crystallization lab (Exp 3). Lab report due: Solubility lab (Exp 2). January 24 Lab: Chromatography (Exp 5), parts A, B, D only. This is a two day lab. Do parts A and B today. Read Chromatography experiment (Exp 5, pp 80-87) and technique references mentioned on p. 80. You will need to read Techniques 12 and 14. Work in pairs for this experiment. Prelab Report: turn in the Chromatography prelab report. January 26 Lab: Chromatography, second lab period, do Part D today. Lab report due: crystallization (Exp 3). January 31 Lab: Distillation (Exp 6A, simple distillation). Read Distillation lab (p 87 - 93) and any technique references mentioned on p 88. Prepare two gas chromatography samples for Charlie. Work in pairs for this experiment. Prelab Report: turn in the Distillation prelab report. February 2 Lab: second day of the distillation experiment, do Part 6A, fractional distillation. Prepare two gas chromatography samples for Charlie. Work in pairs for this experiment. Lab report due: chromatography (Exp 5). February 7 Lab: Isolation of Caffeine from Tea (Exp 11), first day. Read pp 119-127 and technique references on p. 125, especially Techniques 7 and 16. Prelab Report: turn in the Caffeine prelab report. February 9 Lab: Caffeine lab, second day, sublimation. Lab report due: distillation lab (Exp 6). February 10 (Friday) Midterm exam in SL 120 February 14 Lab: Preparation of a C-4 or C-5 Acetate Ester (Exp. 52). This is a two day lab. Read pp 472-475. You will be assigned an ester to prepare. Read the essay on pp 128-130. Read Techniques 7, 8 and 10. We will be doing the infrared spectrum and obtain the gas chromatogram on our product. We might be doing the NMR spectrum on your product: read Technique 19 (pp. 742-745), Appendix 3 and 4. Prelab Report: turn in the C-4/ C-5 Acetate Ester prelab report. February 16 Lab: second day of ester lab, distill product, determine the infrared spectrum, and prepare gas chromatography sample and NMR sample for Charlie to run. Note: we may pass out the proton NMR spectrum for your ester and starting alcohol. Lab report due: caffeine lab (Exp 11). February 21 No lab today February 23 Lab: Competing Nucleophiles (Exp 19A, Exp 19B and 19C) Read pp 194-202 and technique references on page 195. Start Exp. 19A as quickly as possible. Once this is refluxing, then go to part Exp. 19B. In Exp. 19A, we will be substituting 1-pentanol and 2-pentanol for 1-butanol and 2-butanol, respectively. You will be assigned either 1-pentanol or 2-pentanol. In Exp. 19B, we will substitute 2-methyl-2-butanol for 2-methyl-2-propanol. Everyone will do part 19B. For part 19C, which is the analysis of the results of part 19A and 19B, you will submit two samples to Charlie Wandler for gas chromatographic analysis (one for 19A and one for 19B). Prelab Report: turn in Competing Nucleophiles prelab report. Lab report due: C-4 or C-5 acetate esters experiment (Exp. 52). February 28 Lab: Nitration of Methyl Benzoate (Exp. 40). Be sure to read pp 342-346. Read or review crystallization procedures in Tech 5, Sections 5.3 and 5.7. Prelab Report: turn in the nitration of methyl benzoate prelab report. March 2 Lab: Nitration of Methyl Benzoate, second day. Run the infrared spectrum of your product. Lab report due: Competing Nucleophiles (Exp 19). March 7 No lab today March 9 Checkout of Laboratory and clean up. You must do a lab cleanup job and check out of the lab on March 9th. Failure to properly check out of the lab will result in a fee charged to you. Equipment must be clean and dry. Line up the equipment in the exact order shown on the check-in list to speed up the check out procedure. Lab report due: nitration of methyl benzoate (Exp. 40). March 15 Final exam: A. Tuesday, March 14th in SL120, 3:30 - 5:30 GENERAL LABORATORY REGULATIONS AND SAFETY CONCERNS (Text: pp. 4-20) It is absolutely necessary that good safety practices be followed in the laboratory, for your good health and mine. The safety quiz is available online. This quiz must be passed. B. 1. Wear safety glasses at all times. If you don't have a pair already, please purchase them from the bookstore. The safety glasses must completely cover the eyes, with no gaps. 2. No open flames are allowed in the laboratory except as noted by the instructor. You can use a water bath, hot plates, or aluminum blocks for heating liquids. 3. Note positions of eye wash fountains, safety showers, and fire extinguisher. 4. Please don't bring food or drinks into the lab. 5. Organic solvents must be kept in the hood. Handle solvents with care and avoid contact with skin (use gloves, if necessary). Don't breathe solvent fumes. Always evaporate solvents in the hood! Most solvents are toxic! 6. Proper disposal of organic solvents: We have waste containers in a hood for proper disposal of organic compounds. Aqueous mixtures should be poured into proper containers, as well. If in doubt as to where to put the stuff, please ask. 7. Dispensing reagents: You should always take a spatula with you when you go to the balance to aid in transfer of solids. A Pasteur Pipet is useful for liquids. Be sure to clean up any spills on the balance pan. Read pages 43-46 and pages 512-518 for help in transferring and measuring liquids. 8. Report all accidents to your instructor, immediately! 9. Students are responsible for their own medical coverage in case of injury. 10. Clean up your laboratory bench and hood before leaving the lab. Be sure to clean the balance. If we do well in cleaning during the quarter our cleanup duties on March 9th will be minimized! Clean up any spills when they occur! 11. You must do your laboratory work during our laboratory periods. Attendance is required unless permission has been obtained in advance from the instructor. No makeup periods have been scheduled! Good lab preparation, in advance of the lab, improves safety! 12. Health Insurance. According to University policy, students are responsible for their medical coverage in case of injury in the laboratory. It is recommended that you obtain medical insurance. If you are not already covered by your family's or your own health insurance policy, you might consider purchasing insurance through the University-sponsored Student Health Plan. For more information, contact Health Services. LABORATORY FEE A laboratory fee of $37.20 has been included as part of the fees that each student must pay as part of tuition and fees. This fee covers normal and minimal breakage as well as costs associated with chemicals and supplies need in this course. The money also helps in costs associated with replacing expensive instrumentation required with doing modern chemistry. Students who break glassware in excess of the normal amount will be required to pay for broken items. Please be careful! C. CHECK-IN TO LABORATORY On January 5th you will be given a check-in list, including a combination number for your lock. Carefully check your equipment against the list (Text: pp. 33-36). If you are missing items, get them on January 5th. After this date, you must pay for anything that is lost. Make sure that you remember to put the clamps away at the end of the period! Be sure to write down your combination number and lock your locker before leaving the laboratory. Many students aren't too careful on this score! Departmental policy requires that student's checkout of the laboratory at the end of the quarter (including mid-term withdrawals). D. PRELABS Complete the prelab report and turn it before the end of the period. E. LABORATORY NOTEBOOK AND REPORTS (PAGES 20-27 OF TEXT) A bound laboratory notebook is required for taking notes and data. The bookstore has several types of notebooks. Pick one that is lined. If the pages aren't numbered, you should number them yourself in ink. Allow the first several pages for a table of contents. Allow an adequate number of pages for the experiment, so that one doesn't have to skip ahead in the notebook. All notebook entries must be made in ink, and all data recorded directly into the notebook at the time of observation. A recommended format is shown in "Advanced Preparation and Laboratory Records" (pp 20-27). I will inspect your laboratory notebook on the day you start the lab experiment. A brief procedure should be included, along with any necessary calculations asked for in the experimental procedure. Report sheets are available from my website for Chemistry 354. Transfer the appropriate information from your laboratory notebook onto the sheets. These reports should be submitted by the due date indicated on the schedule. The report sheets include spaces for answers to the assigned questions. The reports must be legible and you must follow acceptable standards of English, including correct spelling. F. SAMPLES (PAGE 27 OF TEXT) Submit samples in a properly labeled vial with the report sheet on the laboratory due dates. See page 27 for label format. G. LABORATORY TESTS (two @ 100 pts each) Midterm exam: Friday, February 10th in SL 120 Final exam: Tuesday, March 14th in SL120, 3:30 - 5:30 H. GRADE IN COURSE The points for the course will be distributed as follows: Examinations (2 @ 100 points each) Laboratory Reports and Technique TOTAL 200 points 200 400 points Note that each experiment is worth 20 points. These 20 points are distributed as follows: Pre-Lab Exercise 5 points Results, answers to questions 15 The total points accumulated laboratory points during the quarter will be multiplied by a scaling factor to establish a 200 possible points for the Laboratory Reports. Grades will be assigned on an absolute scale of points according to the following standards. A B C D F 90 to 100% 80 to 90% 65 to 80% 55 to 65% below 55%