Specific Heat Lab
advertisement

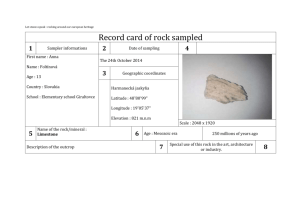
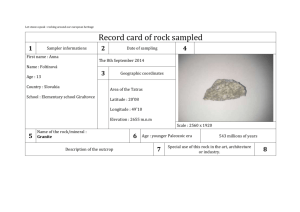
Are they selling Granite? Introduction The specific heat capacity is the amount of energy required to change the temperature of one gram of a substance by one Celsius degree. Each substance has a unique specific heat capacity. In this lab you will use a determination of the specific heat capacity to identify an "unknown" rock sample. Purpose To determine if the sample of rock that you are given is granite by measuring the specific heat capacity Materials Safety goggles Lab apron Calorimeter Graduated cylinder hotplate Tongs Stirrer Thermometer Safety 1. Thermometers are fragile. Be careful in handling them, and never use a thermometer as a stirring rod. If you are using a mercury thermometer and it breaks, notify your teacher immediately. Mercury vapors are poisonous. Procedure 1. Put on your lab apron and safety goggles. 2. Measure EXACTLY 300.0 mL water and place it in the calorimeter. Record the temperature of the water. 3. Record the temperature of the boiling water bath and carefully remove a rock sample from the boiling water bath with a pair of crucible tongs. 4. Quickly place the rock sample into the calorimeter being careful not to overflow it. Close the lid tightly and record the temperature every 30 seconds for about 5 minutes. The temperature should first rise, level, and then fall. The maximum recorded temperature will be used in the calculations of specific heat capacity. 5. Remove the rock sample from the calorimeter and place it on the counter to cool. Record the number listed on the rock sample and refer to the chart to determine the mass of the sample. Record this value in Table 1. 6. Empty the calorimeter and repeat steps 2-5 with another rock sample from the boiling water bath. 7. Empty the calorimeter and gently place the rock samples back into the boiling water bath. Add more water to the water bath if necessary. *Skip this step if this is the last class of the day. Cleaning Up 1. Return all materials to their proper locations. 2. Wash your hands thoroughly before leaving the laboratory. Data Presentation TABLE 1. Calorimetry Data Table Trial #1 Trial #2 Initial temp. of water in calorimeter Initial temp. of rock (boiling water bath) Final temp. of water in calorimeter (max temp) Rock # and mass from chart Mass of water in calorimeter (1ml = 1g) TABLE 2. Calculation Summary Trial #1 Trial #2 Heat transferred to the water Specific Heat Capacity of Rock % error Data Analysis Specific Heat Capacities (J/g°C) Water: 4.184 J/g°C Granite: 0.803 J/g°C 1. Make a temperature versus time graph (hand drawn is acceptable) showing the temperature data collected in step 4 of the procedure. There should be 2 lines (one for each trial). Indicate the final temperature (max temp) of the rock that will be used to calculate the specific heat capacity. 2. Use the formula q = m s t to calculate the heat transferred to the water in the calorimeter in each trial. Show the calculations and record the values in Table 2. 2. How does the heat transferred to the water in the calorimeter compare to the heat transferred from the rock in the calorimeter? 3. Use the formula q = m s t to calculate the specific heat capacity (s) of the rock in each trial. Show the calculations and record the values in Table 2. 4. Assuming the identity of your rock is granite, what is the percent error in the specific heat capacity? Show the calculations and record the values in Table 2. % error = [(experimental s – known s)/known s] X 100 Conclusions 1. How is the final temperature of the rock determined? 2. Why is a hot water bath used to heat the rock? 3. Why is the volume of the water in the calorimeter measured when the mass of the water is needed for the calculations? 4. Both of the following errors would cause a change in the calculated specific heat capacity for the rock. Tell if the change would be to raise or lower the calculated value of the specific heat capacity. Explain. a. A significant amount of water is transferred with the hot rock. b. The rock "cools off" as it is transferred from the hot water to the calorimeter. 5. Suppose that in the procedure a rock sample at room temperature was added to hot water. How would this affect the percent error (higher, lower, or the same)? Explain the reasoning. 6. Explain if the results would be similar if a beaker was used instead of the calorimeter? 7. Do the data support or refute the claim that this rock material is composed of granite? Explain the likelihood of the data to convince someone that the company is indeed selling granite. TABLE 1. Rock Numbers and Corresponding Masses Rock # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Mass (g) 103.36 124.61 182.26 86.60 119.01 157.57 140.50 156.10 125.09 63.14 123.02 74.27 89.40 119.16 82.76 103.33 131.12 111.84 116.90 95.54 137.41 76.82 59.19 57.88 58.10 56.99 59.01 36.53 159.74 ---51.27 171.00 163.81