Protocol #1: Gram Staining Instructions
advertisement

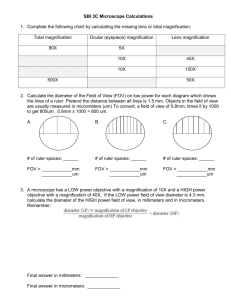
Bacteria Lab After this lab a student will be able to: 1. perform a competent gram stain and diagnose the result 2. identify the major shapes of bacterial cells 3. demonstrate the use of oil immersion for visualizing bacterial samples Part 1. The Gram Stain. You will perform a gram stain on 2 different cultures provided in the lab. You will sketch and describe the results for both of the samples and write an SOP (Standard Operating Procedure) for doing the gram stain. For an SOP you write down exactly what you did in the procedure not necessarily what you manual or text says. So there are 2 sets of directs below, choose the protocol that best fits your lab materials or your understanding, modify if necessary and write down what you did. Protocol #1: Gram Staining Instructions 1a. BROTH: Apply 1 or 2 loopfuls of broth to a clean slide. 1b. PLATE: Place a drop of tap water onto a slide. Touch a colony with a loop and mix the bacteria with the water until there is a uniform, thin film of microorganisms on the slide. 2. Air dry and heat fix the slide by waving it a few times using Bunsen burner. Allow the slide to cool. 3. Stain with Crystal Violet for 1 minute. Rinse with water. 4. Apply Iodine solution for 1 minute. Rinse with water. 5. Decolorize by adding gram's decolorizer (alcohol or acetone) drop by drop until the purple stops flowing. Wash immediately with water. 6. Counterstain with Safranin for 30 seconds. Rinse with water. 7. Blot gently/Air dry the slide. Observe using oil immersion microscopy From: http://student.ccbcmd.edu/courses/bio141/labmanua/lab6/lab6.html Protocol #2: PROCEDURE 1. Heat-fix a smear of culture as follows: a. Using the dropper bottle of distilled water, place a small drop of water on a clean slide by touching the dropper to the slide (see Fig. 1). b. Using a disposable loop, aseptically remove a small amount of the culture from the agar surface, or a drop from the tube culture and just touch it several times to the drop of water until it just turns cloudy (see Fig. 2). c. Using the loop, spread the bacteria/water mixture over the entire slide to form a thin film (see Fig. 3). d. Allow this thin suspension to completely air dry (see Fig. 4). e. Pass the slide (film-side up) through the flame of the bunsen burner 3 or 4 times to heat-fix (see Fig. 5). 2. Stain with crystal violet for one minute (see Fig. 6). Gently wash with water (see Fig. 7). Shake off the excess water but do not blot dry between steps. 3. Stain with gram's iodine solution for one minute (see Fig. 8) and gently wash with water. 4. Decolorize by adding gram's decolorizer (alcohol or acetone) drop by drop until the purple stops flowing (see Fig. 9). Wash immediately with water. 5. Stain with safranin for two minutes (see Fig. 10) and wash with water. 6. Blot dry and observe using oil immersion microscopy (see next section) More on gram staining and diagnosis: http://www.bact.wisc.edu/Microtextbook/index.php?module=Book&func=displayarticle &art_id=89 Gram Stain tutorial: http://www.ncl.ac.uk/dental/oralbiol/oralenv/tutorials/gramstain.htm Gram Stain movie (good sound track): http://video.google.com/videoplay?docid=5961914877736413646 Using the Oil Immersion Lens View the bacteria first through the 40X lens and then through the oil immersion lens. To use the oil immersion lens proceed as follows: Focus the 40X lens on the sample and examine it. o Decrease the amount of light if you are having difficulty seeing something. Turn the barrel of the microscope so that the 40X and 100X lens is 1/2 way between and directly over the sample. Place a drop of oil on the middle of the sample. Carefully turn the objective lens until the 100X lens contacts the oil and snaps into position. Examine the lens; it should almost touch the sample or coverslip and the oil should fill the space between the coverslip and the lens: Fig. 3 C. If it touches the slide or coverslip carefully back the lens off until it is about 1 mm above. While looking through the eyepiece, use the FINE ADJUSTMENT knob to move the lens up and down in tiny increments; this action should bring the organisms into focus. o If you can't see anything initially try varying your lighting. o If you are still having trouble seeing something try to center a piece of debris directly under the lens by eye and then focus on it. Once that is done carefully move away and examine other areas of the slide. Video on microscope use and care: http://biology.clc.uc.edu/Courses/bio111/microscope.htm Part 2. Bacteria Diversity : Now that you have mastered the gram stain and oil immersion obtain, prepared slides of the 3 bacterial shapes and sketch and label them with scale. For each sample indicate the size of the bacterial cells. There are also 3 types of cyanobacteria you will have to make wet mounts, sketch, label and determine the cell size of these as well. Part 3. Experiment on the Mutation Rate in Bacteria (Week 1) Introduction. When thinking about the rapid rate at which antibiotic-resistant bacteria become selected for in nature, it is important to remember how very small bacteria are and how relatively small volumes can hold astronomical numbers of bacteria. As an example, the "normal" mutation rate for a gene in nature is about one mutation in every million to every billion divisions. Let's assume then that only one bacterium in 100,000,000 has a mutation in a particular gene. (A mutation rate of 1/100,000,000.) If we compared this with the population of the United States, approximately 266,000,000, that would be equivalent to only 2 or 3 people in the whole country having that mutation. The population of the world is estimated to be approximately 6, 500,000,000. At a mutation rate of 1/100,000,000, only 65 people in the world would exhibit that mutation. However one milliliter of bacteria (about 10 drops) contains approximately 1,000,000,000 bacteria. This is approximately 3.4 times the population of the United States! The tube below containing 6.5 ml of bacteria growing in broth has a number of bacteria approximately equal to the number of humans on this entire planet! Assuming approximately 1,000,000,000 bacteria per ml, that means that 10 bacteria in each milliliter from that tube would have a mutation in that one particular gene. Furthermore, since a typical bacterium has in the neighborhood of 3500 genes, that means that each milliliter of that culture contains approximately 35,000 mutations! From http://student.ccbcmd.edu/courses/bio141/lecguide/unit2/control/mutate.html Procedure: In this experiment you will be testing the response/ survivorship of a bacterial population first to an application of a disinfectant at 3 different concentrations (serial dilutions) and then to 3 different concentrations of antibiotics. In each case we can estimate the number of mutants in the population that are resistant to the treatment. Your instructor may decide to put different lab teams in charge of different types of treatments and compile the results from all groups at the end of the experiment. You will make 3 serial dilutions of the disinfectant (see next section) and then inoculate each with a loop full of bacteria from your assigned culture. Then you will mix thoroughly and let sit for 15 minutes (this timing is the common recommendation for disinfecting tables etc.) Then remove 0.1 ml from each tube using a pipette and spread on a nutrient agar plate. Tape the plates shut and label the treatments, your group name, lab day, and instructor name. Next week you count the number of colonies on each of the plates to determine the bacterial count in each of the treatments. Generally if there are 8 teams will have 3 replicates of each type of bacteria and 2 groups should perform a serial dilution of the bacterial cultures themselves to allow a control for the bacterial counts in each of the cultures, because we will need to know how many bacteria are present per ml in the original cultures. General Serial Dilution Protocol Successive Serial Dilutions The technique used to make a single dilution is repeated sequentially using a more and more dilute solution as the "stock" solution. At each step 1ml of the previous dilution is added to 9ml of distilled water. Each step results in a 10 fold change in the concentration from the previous concentration. The values shown in the tubes are the amount of stock present in each ml of dilute solution. The dilution of the original stock solution is shown below the tubes. From: http://biology.kenyon.edu/courses/biol09/tetrahymena/serialdilution3.htm More on serial dilutions: http://abacus.bates.edu/~ganderso/biology/resources/dilutions.html Short video on serial dilutions and bacterial count: http://education.wichita.edu/saltymicro/ecology_interactives/Serial%20DilutionREV.pdf Serial Dilutions worksheet: http://faculty.stonehill.edu/calmeida/pdfs/micro_ca2.pdf Serial Dilution tutorial: http://biology.clc.uc.edu/Courses/bio111/yst-plt.htm Appendix A: Fundamentals of Microscopy Objectives When you finish this worksheet you should be able to: 1. Define these terms and use them in an appropriate context: a. diopter value b. FOV c. interpupillary distance d. parfocal e. resolution 2. Carry a microscope safely to your desk. 3. Name the parts of the microscope and their functions. 4. Discuss the advantages and disadvantages of using compound microscopes and dissecting scopes. 5. Calculate interpupillary and diopter values. 6. Prepare dry and wet mounts for use with the compound microscope. 7. Correctly focus the microscope on tissue and be able to calculate cell size. 8. Clean the microscope and correctly prepare it for storage. Introduction Many organisms are too small to be observed with the naked eye. These organisms are multicellular as well as single celled. Representatives of all five phyla, plants, animals, fungi, protists, and bacteria are microscopic in size. Microbes are less than 1 millimeter in size and usually measured in micrometers (1 um=1/1000 mm.) Use of the Dissecting Scope Today in lab, we will begin by using the dissecting scope which magnifies objects as much as a magnifying glass. The field of view for this device is large and the depth of field that is in focus at one time is also large. Light can be directed from above the object or below. Magnification is controlled by a zoom knob. *Carry the dissecting microscope by its arm with one hand supporting the base. After placing it on the table, name the parts and associated functions. Parts of the dissecting scope (Fig. la)(back Page) Base with illuminator. Light switch: Turns on the illuminator. Illuminator control knob: Two positions for one switch may alternately select lighting from above or below the stage or both lighting sources may be used simultaneously. Oculars: Move them together or apart until you feel comfortable looking into the microscope. A single circle of light should be observed. Zoom knob: Controls magnification of the specimen. Rotate it to increase or decrease Cleaning the Lenses Nothing should touch the microscope lenses except the special lens paper and appropriate solvent. The lenses may be cleaned with clean lens paper. Lens cleaner must be used to clean grease or oil on the lenses. Handle solvents carefully and wash your hands after use. Calculating Interpupillary Distance and Diopter Values. Before viewing a slide one should calculate the interpupillary distance using the following steps: (1) Focus on a specimen using the low power objective. (2) Sit back from the microscope and slowly move the oculars apart. (3) While attempting to look through the oculars, notice that you can see two images. Carefuly move the oculars together until the split image is viewed as one. (4) Repeat the procedure. (5) Locate the scale on the microscope between the oculars. Record the number in your lab book. The diopter values must also be calculated: (1) After the interpupillary distance has been determined, locate the diopter adjustment rings on the ocular lenses. (2) Turn the diopter rings so that the mark on each ring aligns with the midpoint of the microscope scale on the ocular. (3) Close the left eye, and use the fine focus to find the clearest possible image. (4) Open the left eye, and close the right eye. (5) Do not change the fine focus, but instead, turn the diopter ring on the left lens from one extreme to the other to find the best viewing place. (6) Repeat the procedure, and record the diopter values in your lab book. (Note: Your interpupillary and diopter values will apply to other microscopes in the lab.) Viewing a Slide Place the slide on the stage, using the stage clips to hold the slide securely. Adjust the light source to provide 75% of the total light. Always begin slide viewing with the lowest power objective available. Click the scanning objective in place and turn the coarse adjustment knob until the sample comes into focus. Then use the fine adjustment knob to refine the image. Adjust the amount of light coming through the sample using the light rheostat and the iris diaphragm lever. While you are looking at the stage move the adjustment knobs to observe whether the objective is moving closer or further from the stage. Turn the mechanical stage drive knobs, one at a time. Note the direction that they move the stage when turned clockwise and counterclockwise. Focus on the sample, change objectives by moving to the next highest objective. The microscope is parfocal, therefore it should not be necessary to adjust the view with the coarse adjustment knob. Use only the fine adjustment knob from this point. Note that the field of view has gotten smaller while the magnification increased. The depth of field also is decreased. Only a thin layer of tissue is in focus at higher magnifications. By focusing up and down you can view different parts of a single cell or different cell layers. The specimen must be centered over the hole in the stage through which light passes. Oil Immersion The resolution of the image is increased by filling the space between the slide and the lens with oil. Oil has a much higher index of refraction than air and contributes to the magnification of the image. 1. Locate the object and center it with scanning lens. 2. Move to low, then to high power, refocus while using the fine adjustment kob only. 3. Swing the high power lens away from the slide. Place a drop of immersion oil on the center of the coverslip and move the oil immersion lens into position. Refocus using the fine adjustment knob only. 4. When you are finished, swing the lens away. Do not put any other lens in position. 5. Remove the slide and wipe most of the oil off with paper, then with lens cleaner. 6. Clean the lens also with lens cleaner and then dry lens paper. Computing Magnification Magnification is important in examination of cells and tissues. It is not the only concern in observation. Objects must not only appear larger, but also clearer. Resolution or resolving power measures how clearly details can be seen. It is the smallest distance between two points which can be separately distinguished as different points. Total magnification using the light compound microscope can be calculated by multiplying the magnification of the ocular lens by the magnification of the specific objective. Table 1.2 Measuring Objects Using the Microscope Remember: as you increase the magnification you are decreasing the FOV (Field of View). If you know the FOV and the magnification when viewing an object you can calculate the size of the cell or organelle that you are observing. There is a mathematical relationship between the magnification and the diameter of the FOV. X, = D2 X2 D, Where X, = Magnification with a specific objective. X2= Magnification using a different objective. D, = Diameter of FOV at magnification 1. D2 = Diameter of FOV at magnification 2. We can therefore calculate any of these values if the other three are available. Such as: 2=P.X1 1t2 Record the diameters of the FOV for all objectives that you are using today in Table 1.2. Measuring cells. Today you will observe cell types presented by your instructor. They may be prepared slides or wet mounts that you have prepared with materials provided for you. Calculate cell size by estimating the number of cells that would fit across the FOV and then divide the diameter of the FOV by the number of cells. cell size = diameter of the FOV number of cells As we estimate the size of the cell that we are viewing we must take into consideration the objective that we are using to view the object with and the diameter of the field of view (FOV.) Using the scanning objective, the diameter of the FOV is 4.5 mm. The diameter of the FOV for low power is 1.8 mm, the diameter of the FOV for high power is.45 mm and the diameter of the FOV for oil-immersion is-.18 mm. For example: if you count 5.5 cells that would fit across the diameter of the field of view using the scanning objective, you would divide 4.5 mm by 5.5 cells giving a cell length of .818181.... (Using the rules in your appendix for significant digits you know that we cannot have more than two significant digits in the answer.) Thus we will round off our answer to .82 mm. To convert this to um we use the conversion factor that relates mm to um. .82 mm X 1000 um = 820 umlmm Use the best objective for viewing the cell. It is not always best to use the highest power objective. (However you are likely to estimate more accurately and you don't have to count as many cells.) Example: 12 cells fit across the diameter of the FOV using low power. Calculate cell size. cell size =,diameter of FQV number of cells across the diameter of the FOV cell size = 1.8 mm 12.0 cells = .15 mm/cell Using low power, calculate the cell size in the following diagram: cell size = diameter of FOV number of cells across diameter of FOV Calculate cell size in the following diagram of a single organism viewed under high power. (Remember that you must estimate how many cells would fit across the diameter f the FOV.) cell size = diameter of the FOV number of cells across diameter of the FOV Preparation of a wet mount. Wet mounts are useful in observing living tissues or cells for characteristics such as movement, color or behavior. Follow these steps: (1) Obtain a slide, coverslip and perhaps a teasing needle. (2) Place a drop of the substance on your slide. (3.) Touch the coverslip to one margin of the drop and slowly lower it. The slide should not have bubbles if you perform this carefully, but they do give you something to focus on! Electron Microscopes Electron Microscopes are not generally available to introductory biology students but you may have the opportunity to observe the use of one. Various types of electron microscopes allow viewers to increase the magnification without losing the resolution of the picture. The view does not become fuzzy with increased magnification. The use of these has increased greatly for study of cell structure and identification. Most larger hospitals have electron microscopists to examine tissue samples on site. Electron microscopes use a beam of electrons as an illumination source. The wavelength of the electron is a hundred thousand times smaller than the wavelength of visible light. Transmission electron microscopes, scanning electron microscopes and probe electron microscopes are all available for more sophisticated study of cell structure and structure of macromolecules. Sample exam Questions 1. Discuss the difference between magnification and resolution. 2. Discuss the relationship between: magnification, diameter of FOV and relative brightness for all objectives 3. Discuss how you would calculate cell size of a single celled organism. 4. Compare and contrast the dissecting and light microscopes. From : http://faculty.valenciacc.edu/tklenk/labs/microslab.htm