Lesson Plan
advertisement

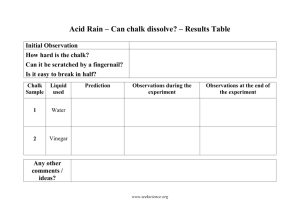
Analyze the impact of natural and human-induced changes to the characteristics and distribution of water in local, regional, and national ecosystems. (Engage, Explore, Explain, Elaborate and Evaluate) Adam Krammer Science Grade 8 Topic: The effects of pollution Class: Grade 8: 8 Date: 2010 Description of Lesson: Students will be able to make connections with why pollution is such an issue in today’s society. They will be doing an experiment and collecting data to explain what is happening and why! Students will do the “Acid Rain” experiment in groups of 3-4 and will need to take pictures as to what is happening. They will be responsible to explore this at a later time. (10-15 min) Students will then move to the computer lab to upload their photos to a file. From here the group will pick a picture that best represents what was happening. They will then use the “Voice Thread” program to show a better understanding of what was happening and why it is relating to real life situations. (40-50 min) Objectives: Knowledge: Developing a deeper understanding of the importance of not littering. They will also be able to better relate why exhaust and other pollutants that enter the air effect the environment. Evaluation: Having students use information gathered by doing the lab experienment they will be able to see the negative effects that acid rain has on buildings and life forms. Students will have to create a Voice Skills/Processes: students will be able Thread presentation in regards to to see why pollutants in the air are what is happening to the piece of such a hazard to our environment. chalk and why this represents real life as well. (Instructions below) Attitudes/Appreciations: Developing a more in depth understanding on why Marks will be given out for this we should be concerned about the air assignment based on the knowledge supply and keeping it clean. They will obtained from the experiment and see that we as humans are making a how the students can relate this back tougher life to maintain when we into real life situations. pollute the air. Materials/Resources: Access to a computer lab with Voice Thread software Jug of vinegar **Lesson Plan idea came from Both Voice Thread Software and Columbia Education Center http://voicethread.com/ http://www.col-ed.org/cur/sci/sci208.txt Analyze the impact of natural and human-induced changes to the characteristics and distribution of water in local, regional, and national ecosystems. (Engage, Explore, Explain, Elaborate and Evaluate) Adam Krammer 4-5 pieces of chalk Safety goggles for each student Procedure: Students will work in groups of 3-4 to preform the experiment taking pictures through the entire process. Once it is completed they will more to the computer lab to upload photos onto a computer. They will then create a Voice Thread in regards to what they learned. (each student must make a comment of 2-3 minutes each) The following link is to Voice Thread website and is free to join and comment on. Please have a look at this so you know what to expect. http://voicethread.com/?#q.b1192.i13696 Lab Activity TITLE: "IT'S EATING AWAY AT YOU" AUTHOR: Judy Grunke, Weiser Middle School; Weiser, Idaho (borrowed from Falma Moye) GRADE LEVEL: TIME: 6-8, Earth or Physical Science 30 minutes--set-up, experiment, and discussion. OVERVIEW: Acid rain is a major pollutant in the world today. This activity is designed to show the effect of acid rain on rock, edifices, and sculptures. PURPOSE: rock. To show the effect of acid rain on limestone OBJECTIVE(s): The student will be able to: 1. Demonstrate, using chalk and vinegar solution, the chemical reaction of acid on limestone. **Lesson Plan idea came from Both Voice Thread Software and Columbia Education Center http://voicethread.com/ http://www.col-ed.org/cur/sci/sci208.txt Analyze the impact of natural and human-induced changes to the characteristics and distribution of water in local, regional, and national ecosystems. (Engage, Explore, Explain, Elaborate and Evaluate) Adam Krammer 2. Explain what happens in this process. 3. Identify the compounds used in the reaction and the gas which is given off. 4. Tell where acid rain originates. 5. Explain the effect of acid rain on limestone buildings and statuary. RESOURCES/MATERIALS: CLASS: Textbook, acid rain FOR EACH LAB GROUP: vinegar solution chalk eye dropper pan to set chalk in and to catch vinegar ACTIVITY AND PROCEDURES: 1. Place a piece of chalk in pan. 2. Using an eyedropper, have one student drop vinegar onto the chalk in a constant stream. 3. Observe the fizzing on the surface of the chalk when the vinegar is dropped. 4. Notice the crumbling of the chalk. TYING IT ALL TOGETHER: 1. Students can research the causes (man-made and volcanic eruptions) of acid rain and efforts to protect valuable buildings and statues. 2. Students can use the vinegar residue to demonstrate what happens to the dissolved material from chemical erosion by placing a small piece of basalt in the vinegar solution. (As vinegar evaporates, the dissolved material from the chalk precipitates to form a white mineral residue--caliche--on the surface.) **• Air pollution is a major cause of acid rain. When rain becomes more acidic than normal it can damage soil, water, building materials, plants, **Lesson Plan idea came from Both Voice Thread Software and Columbia Education Center http://voicethread.com/ http://www.col-ed.org/cur/sci/sci208.txt Analyze the impact of natural and human-induced changes to the characteristics and distribution of water in local, regional, and national ecosystems. (Engage, Explore, Explain, Elaborate and Evaluate) Adam Krammer animals, and humans. • Chalk is made from calcium carbonate (CaCO3)/calcite. • In this activity the vinegar, which is acidic, reacts with the chalk forming carbon dioxide gas (the bubbles you see during the reaction are the carbon dioxide gas forming), water, and another calcium compound. • The remaining material no longer looks like the original chalk. • The chalk remains the same in the water. • This simple experiment demonstrates the effects of acid rain on limestone or calcium carbonate-containing statues and buildings. • The acid rain dissolves the calcium carbonate causing the surfaces to become rough and the intricate detail of the statue/building to be lost." Source and further information: http://peer.tamu.edu/NSF_Files/tk.asp?file=AcidRain1_resultstable||doc&id =185 http://www.answerbag.com/q_view/1302669 **Lesson Plan idea came from Both Voice Thread Software and Columbia Education Center http://voicethread.com/ http://www.col-ed.org/cur/sci/sci208.txt