Name Block ______ Effects of Ocean Acidification: A Look at
advertisement

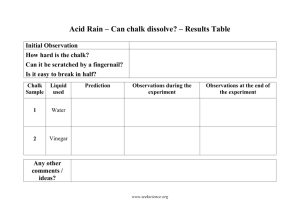
Name ___________________________________________ Block ____________ Effects of Ocean Acidification: A Look at Controlled Experiments The world’s oceans are in trouble. Every day, 22 million tons of carbon dioxide (CO2) from factories, cars, power plants and other human sources are absorbed by the world’s oceans. The result? A frightening phenomenon that's making seawater more acidic, meaning disaster for many marine animals, from plankton and coral up the food chain to sea stars, salmon, sea otters, whales — and ultimately people, who rely on oceans for food. Together we will do an experiment using chalk as coral and vinegar as acid in the oceans to test the affects of acid on living organisms such as coral. Problem: ____________________________________________________________________________________ Write a hypothesis that we can test: If……then……because…. ____________________________________________________________________________________ ____________________________________________________________________________________ ____________________________________________________________________________________ What is the control? __________________________________________________ Name at least 4 constants: 1. _________________________________________________________________ 2. _________________________________________________________________ 3. _________________________________________________________________ 4. _________________________________________________________________ What is the independent variable in this experiment? ____________________________________________________________________________________ What is the dependent variable in this experiment? ____________________________________________________________________________________ Materials: vinegar water chalk graduated cylinder Beakers or containers Goggles Masking tape and pen (for labeling containers) Two strips of litmus paper. Balance (might have to share with other groups) Procedure: 1. Using the masking tape and pen label 1 beaker “vinegar” and 1 beaker “water”. 2. Pour 100 ml of vinegar into the vinegar beaker. 3. Pour 100 ml of water into the water beaker. 4. Using the pH strips, record the pH of the water and vinegar. 5. Record the mass (in grams) of the chalk. Place in the water beaker. 6. Record the mass (in grams) of the other chalk. Place in the vinegar beaker. 7. Swirl the beakers for 10min (to simulate waves). Record at least 3 observations of the chalk in each solution during this time. 8. Record ending mass of both pieces of chalk. Data: Water pH Start chalk mass End chalk mass Change in mass Observations – Chalk in water: 1. 2. 3. Observations – Chalk in vinegar: 1. 2. 3. Vinegar Conclusion Questions: TYPE and submit to apereira@ctriveracademy.org 1. 2. 3. 4. 5. 6. 7. 8. Restate your problem and hypothesis. Was your hypothesis correct? Describe what happened to the chalk in the acidic solution. Why do you think this happened? Would you consider your data to be reliable? Could somebody follow the same procedure and get similar results to yours? Is there anything that you would do to improve this experiment? Do you think that acid has this effect on all sea life? Explain why or why not. What would you tell the EPA about how acid affects coral? Why might marine biologists and other scientists be interested in doing a test like this one?