Syllabus Fall 2015

Instructor: Dr. Bryan G. Splawn
Meeting Place: RMSC 328
Instructor Office Hours: open-door policy
Course Number and Title: CHEM 411,
Instrumental Analysis
Meeting Time: TTh 1:00 – 2:20 pm
Instructor email: splawnbg@wofford.edu
Website: http://webs.wofford.edu/splawnbg/ Instructor Campus Phone: ext. 4669
Course Description:
Instrumental analysis is a study of modern instruments that can identify and quantify almost any gas, liquid, or solid sample. Students taking this course will study various electrochemical methods, spectroscopic methods, chromatographic methods, and mass spectrometry.
Course Goals:
Overarching Goals (higher-order thinking skills):
1.
Solve conceptual and mathematical problems related to instrumentation.
2.
Predict analyte electrochemical, spectral, and chromatographic properties preanalysis.
3.
Measure and interpret absorption and mass spectra, chromatograms, and voltammograms.
Ancillary Goals (lower-order thinking skills):
1.
Perform external standard, internal standard, and standard addition calibrations.
2.
Operate numerous instruments.
3.
Work in teams.
Assessment of Course Goals:
A student’s proficiency of above goals will be assessed by closed-book exams, homework assignments, lab experiments, and ACS cumulative exam.
Technology Skills:
Students will learn how to operate modern instrumentation, such as potentiostatglavaniostat, visible spectrophotometer, fluorometer, gas chromatograph, high performance liquid chromatograph, and mass spectrometer. Moreover, students will learn associated instrumental software that automates above instrumentation.
Instructional Format:
The instructional format for this course is a combination of lecture-discussion, studentcentered learning groups, and laboratory-centered partnerships.
Absence Policy:
Attendance is mandatory to all scheduled lectures and laboratories (see course schedule below). Exceptions are personal illness, family emergency, and athletic events for athletes only. Please notify the instructor as soon as possible if any of these situations arise. Failure to notify and make arrangements with the instructor will result in a grade of zero for exams, homework, and labs missed. No makeup exams, homework assignments, labs or final exam will be given. However, students with an excused absence will be given the opportunity to makeup an exam, a homework assignment or a lab. In addition, students with an excused absence have the option to receive a prorated lab grade for a missed laboratory period.
Statement for Academic Integrity:
Honor Code: All work in this class is conducted under the Honor Code of Wofford College
( http://www.wofford.edu/studentLife/honorCode.pdf
). Any case of academic dishonesty will be dealt with to the fullest extent of the honor code. Texting and emailing in class is strictly prohibited.
Names of Texts and related course materials:
Lecture Text: Harris, D.C., (2007) Quantitative Chemical Analysis, 7th ed., W.H. Freeman and
Co., New York, NY.
Other Materials: Students taking this course need a laboratory notebook (duplicate pages), a pair of laboratory goggles, and a scientific calculator that is non-programmable (no alphanumeric functionality).
Policies on homework assignments (due dates, type and format (oral reports, essays, papers, multimedia project, etc), late assignments, weighting of graded components, grading scale).
Exams: You are required to take four closed-book exams during scheduled lecture periods
(see course schedule below). Each is worth 50 points. The exam format is multiple-choice, short answer, and mathematical problems.
Homework Assignments: You are required to complete all assigned homework, which will be checked at the beginning class. The instructor is not checking for right or wrong answers. Instead he is checking for completion of the work. Incomplete, half complete, or late work will receive a grade of zero (0 points). Each assignment is worth 5 points and due the following lecture period.
Laboratory Experiments: You are required to complete all laboratory experiments. All laboratory reports will be in quadchart format (see course webpage for template). Each quadchart is worth 15 points.
Literature Review: You have an opportunity to search online for a peer-viewed article, write a one-page summary about it, and present it to the class in the form of a creative
YouTube Video or PowerPoint presentation. The following topics must be used to select your article: scanning tunneling microscopy, capillary zone electrophoresis, MALDI mass spectrometry, Raman spectroscopy, electronic nose, biosensors, and lab-on-a-chip devices.
A sign-up sheet will be provided for you to select a topic and a presentation date. If we have a large class, then there will be two students per one topic. If the class is small, then all presentations are individual. Be as creativity as humanly possible and limit our presentation or video to 5 minutes. This assignment is worth 100 points or 15% of your total grade, so take this assignment seriously. Find at least three articles on your assigned topic for instructor approval. Only one article will be selected, and the others will pose as backups.
Extra Credit Tutorials: We have on-line tutorials for both atomic absorption spectroscopy and gas chromatography-mass spectrometry. Those attempting these assignments must provide a printout of completed on-line quizzes. Each quiz is worth 5 addition points; however, you are limited to a total of 25 points.
Final Exam: Students are required to take an ACS cumulative final exam, which is all multiple-choice.
Weighting of Graded Components: Point allocation and grade percentage for each assessment item is posted below.
Assessment Items Points Grade Percentage
Exams (4)
Homework (20)
200
100
32%
16%
Lab Experiments (8) 120
Literature Review 100
Final Exam
Total
100
620
20%
16%
16%
100%
Grading Scale: Your grade in this course is calculated by taking your total number of points earned in the assessment items above, dividing by total number of points possible (620 pts) and multiplying by one hundred to compute a percentage. See the table below for the percent distributions and corresponding letter grades.
Percent Distribution Grade Percent Distribution Grade
100 –94
93 – 90
89 – 87
A
A–
B+
76 – 73
72 – 70
69 – 55
C
C–
D
86 – 83
82 – 80
79 – 77
B
B–
C+
Below 55 F
Course Schedule:
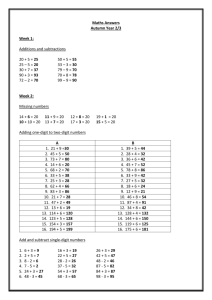
Week 1
8/31 M Fall Semester Begins
9/1 T
T
1-2:20p
9/3 Th 1-2:20p
Lecture: Introduction
HW: CH 5 problems 14, 18, 19, 5-A
2:30-5:30p Lab: Safety Quiz, Lab Check-in
Lecture: CH 5 Quality Assurance and Calibration Methods
HW: CH 5 problems 30, 31, and 5-B
Week 2
9/8 T
T
1-2:20p
2:30-5:30p Lab: Analysis of Copper by Standard Addition
9/10 Th 1-2:20p
Lecture: CH 5 Quality Assurance and Calibration Methods
HW: CH 22 problems 16, 28, 33, 34, 35, 38, 40
Lecture: CH 22 Introduction to Analytical Separations
HW: Study for exam
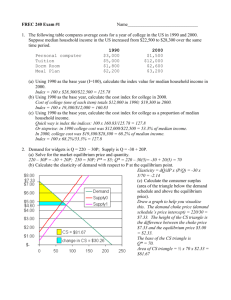
Week 3
9/15 T
T
1-2:20p Lecture: Exam 1 (CH 5 and 22)
HW: CH 23 problems 1, 2, 4, 8ac, 9a
2:30-5:30p Lab: Investigating Gas Chromatography
9/17 Th 1-2:20p
Week 4
9/22 T
T
1-2:20p
Lecture: CH 23 Gas Chromatography
HW: CH 23 problems 5, 20, 21
Lecture: CH 23 Gas Chromatography
HW: CH 24 problems 1, 4, 5, 16a-d
2:30-5:30p Lab: HPLC: Separation of Diet Soft Drinks
9/24 Th 1-2:20p Lecture: CH 24 High-Performance Liquid Chromatography
HW: CH 24 problems 15, 27, 28, 29, 37, 38, 41
Week 5
9/29 T 1-2:20p Lecture: CH 24 High-Performance Liquid Chromatography
HW: CH 14 problems 1, 2, 6, 12, 14
T 2:30-5:30p Lab: HPLC: Separation of Diet Soft Drinks
10/1 Th 1-2:20p Lecture: CH 14 Electrodes and Potentiometry
HW: Study for exam
Week 6
10/6 T 1-2:20p Lecture: Exam 2 (CH 23-24)
HW: CH 14 problems 19, 30, 32, Example p.326
T 2:30-5:30p Lab: Measuring Vitamin C in Orange Juice via CV
10/8 Th 1-2:20p Lecture: CH 14 Electrodes and Potentiometry
HW: CH 16 problems 3, 4, 5, 11, 16, 17
10/9 F N/A Fall Break
Week 7
10/13 T
T
1-2:20p
10/15 Th 1-2:20p
Lecture: CH 16 Electroanalytical Techniques
HW: CH 16 problems 23, 33, 37
2:30-5:30p Lab: Measuring Vitamin C in Orange Juice via CV
Lecture: CH 16 Electroanalytical Techniques
HW: Study for exam
Week 8
10/20 T
T
1-2:20p
10/22 Th 1-2:20p
Lecture: Exam 3 (CH 14 and 16)
HW: CH 17 problems 4, 7, 8, 9, 10, 16; CH 18 problem 1
2:30-5:30p Lab: Spectrophotometric Analysis of Gatorade
Lecture: CH 17 Fundamentals of Spectrophotometry
HW: CH 17 problems 24, 25, 27, 28, 30
Week 9
10/27 T 1-2:20p Lecture: CH 17 Fundamentals of Spectrophotometry
HW: CH 19 problems 1, 3, 4, 6, 7, 19-B
T 2:30-5:30p Lab: Concepts in Molecular Fluorescence
10/29 Th 1-2:20p Lecture: CH 19 Spectrophotometers
HW: CH 19 problems 30, 31, 33
Week 10
11/3 T 1-2:20p Lecture: CH 19 Spectrophotometers
HW: CH 20 problems 2, 3, 4
T 2:30-5:30p Lab: Concepts in Molecular Fluorescence
11/5 Th 1-2:20p Lecture: CH 20 Atomic Spectroscopy
HW: Study for exam
Week 11
11/10 T 1-2:20p Lecture: Exam 4 (CH 17 and 19)
HW: CH 20 problems 1, 5, 6, 9, 15, 16
T 2:30-5:30p Lab: Atomic Absorption of Copper in Vitamins
11/12 Th 1-2:20p Lecture: CH 20 Atomic Spectroscopy
HW: CH 20 problems 21, 24
Week 12
11/17 T 1-2:20p Lecture: CH 20 Atomic Spectroscopy
HW: CH 21 problems 1, 2, 4, 5, 19, 21
T 2:30-5:30p Lab: Atomic Absorption of Copper in Vitamins
11/19 Th 1-2:20pm Lecture: CH 21 Mass Spectrometry
HW: CH 21 problems 8, 9, 12, 13a
Week 13
11/24 T 1-2:20p Lecture: CH 21 Mass Spectrometry
HW: CH 21 problems 23, 25, 26, 21-H, 21-G
T 2:30-5:30p Lab: GC/MS: Separation and Identification of Fatty Acids
11/25 W N/A Thanksgiving Break
11/26 Th N/A
11/27 F
Week 14
N/A
Thanksgiving Break
Thanksgiving Break
12/1 T
T
1-2:20p Lecture: CH 21 Mass Spectrometry
HW: Study for exam
2:30-5:30p Lab: GC/MS: Separation and Identification of Fatty Acids
12/3 Th 1-2:20p
12/4 F N/A
Lecture: Discuss Final Exam; Lab Check-out
Last Day of Classes
Week 15
12/9 W 9-12noon ACS Final Exam
12/14 M 5:00p Final Grades Due
Note: Syllabi subject to change upon notice.
The instructor reserves the right to change any part of this syllabus during the semester, if deemed necessary. When changes are made, they will be announced in class.