LabBufferTitrationVDS_Spring13
advertisement

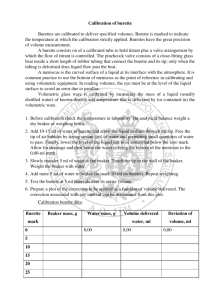
Virtual Drug Screening stream Spring 2013 Lab: Buffer Titration Objective The objectives of this lab are to use a burette to titrate a buffer; to determine the pKa of a buffer; and to implement the Henderson-Hasselbalch equation to calculate the pH of a buffer. You will also determine how precise and accurate your experimental data is. (paraphrase this for your notebook) Hypothesis: formulate your own hypothesis for this lab based upon the dependent and independent variables. Your hypothesis must be testable within the limitations of the experiment. Pre-Lab Skim this handout and read the instructions on the last 3 pages on how to calibrate these pH probes and how to use a burette. Answer the following pre-lab questions: 1) What are buffers made up of? Why are buffers useful? Give examples of when a buffer is useful in lab experiments as well as in living organisms. 2) Include the molecular structure of Tris. Write out the chemical formula of Tris and calculate the formula weight. What is the ‘real’ pKa of Tris – where did you look this info up? What is the buffering range? 3) What is your ‘titrant’ in this experiment? 4) Why do we perform a calibration? Lab Safety You will be handling strong bases this week and lots of glassware so please wear appropriate clothing, safety goggles and gloves at all times. Read through the Lab protocol to make sure that you understand all the procedures before you start your Lab. Halfequivalence Point 15ml Equivalenc Point 30ml Background Figure 1 is an example of the titration curve that you will obtain from your Figure 1. Generic titration curve Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 1 Virtual Drug Screening stream Spring 2013 buffer titration experiment. The equivalence point here is arbitrarily set at 30ml as an example. This is the upper limit of the buffering capacity of your buffer where a small volume of added base will increase the pH of your buffer by a lot. If you add more base beyond the equivalence point, you will see that the pH slowly plateaus out. This is not shown in Figure 1 due to the range of added base and acid presented here. The half-equivalence point is then determined by dividing the volume of base at the equivalence point by 2. The pH at the half equivalence point is the pKa of your buffer of interest. Procedure: This lab should take you 3.5 - 4 hrs to complete. Work in pairs. 1) Obtain the following equipment and glassware: 1- 50ml burette (see TA/mentor) 3- glass beakers (1 x 100ml, 2x 250ml) 1- stirring plate with a rod attached to the 1- magnetic stir bar back of the stirring plate or a separate rod 2- graduated cylinder (~250ml and 100ml) and stand 2- Appropriate volume pipettors to measure 2- clamps (for burette and pH probe) out your stock solution 1- Labpro pH probe and Labpro interface 1- rubber bulb pipettor 1- computer with Logger P 2) Prepare the following solutions and place each solution into an appropriately labeled 250ml container (either beaker or bottle): Your titrant solution: 250ml of 0.2M NaOH Make this by diluting the 5M NaOH stock that you made in the first lab. Please show your calculations for the volume of water and the volume of stock solution to add for preparing the 0.2 M NaOH. You can use the following equation: C1 x V1 = C2 x V2 Note: Since your V1 will be a significant proportion of the final volume, you should define V2 in terms of V1 (If this doesn’t make sense – ask a mentor to help explain) Your buffer solution: 200ml of 0.1M Tris. You will be doing 3 titrations, so 50ml of the 0.1M Tris buffer will be used for each titration and there will then be some leftover in case you need to re-do one. Place a stir bar in the beaker containing the 0.1M Tris buffer solution. Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 2 Virtual Drug Screening stream Spring 2013 3) Prepare your burettes and set them up with the burette clamp and the stir plate as in the figures 2 and 3 below. Please handle the burettes gently and pick the ones that are not chipped. Follow the instructions in the Burettes and Titration handout (at the end of this handout) to prepare the burette and set up the apparatus as in Figures 2 and 3 below: Figure 3 Figure 2 4) Set up the LabPro pH meter system with the LabPro interface as seen in figures 2 and 3. The pH meter should be connected to channel one of the Labpro interface and the Labpro interface can be connected to the computer using the USB cord. The LabPro interface is powered by a DC adapter and needs to be plugged into an electric socket. A small green light should be blinking on the LabPro interface to indicate active connection between the pH probe and the computer controller. 5) Follow the instructions in the “How to measure pH with the LoggerPro”, appended to the end of this lab handout, to run the software and calibrate your pH meter. You can calibrate the pH meter with the pH 7 and pH 10 standard buffer solutions because those values are close to the range of our pH buffer for this experiment (Tris). The standard buffer solutions are colored solutions (pink, blue, yellow) that are located by the weighing balance. NOTE: please be sure to rinse your probe before transferring between the pH 7 and pH 10 standards – we don’t want to contaminate these stocks Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 3 Virtual Drug Screening stream Spring 2013 a) On the desktop of the computer, start the “LoggerPro” program by double clicking on the icon. b) The pH probe is an “auto-ID sensor” (i.e. is automatically detected and recognized). The sensor will be identified, and default data-collection parameters loaded. Perform the calibration using guidelines described in the handout in the last few pages of this protocol. (be sure to include these steps in your lab notebook) c) In “Experiment” menu, click on “Data Collection”. In the pull down menu of “Modes”, select “Events with Entry”. This selection will prompt you to enter a new column title. Since in the titration experiment we need to plot pH vs. Volume of titrant, a new “Volume” column needs to be generated here and given units. d) Save this experiment file with your name (as a .CMBL) in the VDS folder on the Desktop for now and online for long term storage. Proper filename format: UTEID_VDS_BufferTitrationLab_DATE.cmbl 6) You are now ready to titrate your Tris buffer solution. a) Prepare a burette by adding about ~40 ml of 0.2M NaOH solution by following the instructions in the Burettes and Titration section appended to the end of this handout. Make sure your burette is not leaking, and is free of air bubbles. b) Read the initial burette level, and enter it on the computer - this should be something like ~50 ml Write down the initial burette reading (Vinitial) _______ ml c) Rinse a calibrated pH electrode with DI (Nanopure) water from a wash bottle, and blot (do not rub) it dry with a Kimwipe. d) Place your beaker containing 50ml of the 0.1M Tris buffer solution on a stir plate underneath the burette. Make sure that the stir bar in your beaker is mixing gently. Immerse the pH probe in the beaker making sure the stir bar does not hit the pH probe. e) Click on the “Start” button on the menu bar of the LoggerPro program, and monitor your pH reading for approximately 5-10 seconds to ensure it is stable. Click on the “Keep” button, a window will pop up to prompt you to enter the initial volume reading that you recorded in step ‘a)’ - enter it on the computer You will be counting down from this initial volume now. Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 4 Virtual Drug Screening stream Spring 2013 What is the starting pH of your 0.1M Tris buffer? _____________ Titration #1 This answer is important for your post-lab questions. Record it for EACH titration you do. f) Record a pH reading for every ~1 ml increment of titrant solution by pressing “Keep” after delivering each increment, and entering the burette volume level counting down from your initial volume. Make sure the pH reading is stable before you click “Keep”. After approximately 10-15 ml of NaOH has been added, the pH will change more quickly (i.e.: it is close to the end point of the titration). Reduce the increment to ~0.5 ml to accurately measure the inflection point. g) Once the end point is passed, continue to add titrant solution with increments of ~2 ml to collect another 3-4 data points to ensure the pH in solution stops rising. h) Click on the “Stop” button once you are done with the 1st titration. 7) When done pour the titrated Tris buffer into the Basic waste container under the chemical fume hood. Rinse out your beaker with diH20 and dry it with a paper towel. 8) You will first analyze this Titration#1 to see that everything is good. Then you will repeat the titration twice to get repeatable data. To analyze your data, follow the instructions below. a. Create a new calculated column in LoggerPro by opening the Data menu, and selecting “new calculated column”. You need to convert the “Volume” column into “Actual volume”, which is achieved by subtracting the initial burette reading (Vinitial) from each volume entry. b. Once the “Actual Volume” column is created, you can easily change the titration plot by single-clicking on the title of the horizontal axis, and select “Actual Volume” from the pop-up list. c. Print out a copy of the titration curve (pH vs. Actual Volume of NaOH). d. You can easily make a first derivative plot on LoggerPro to help you identify the equivalence point of your titration curve. To do this: add a “New Calculated Column” from the “Data” menu, and name it “Derivative” Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 5 Virtual Drug Screening stream Spring 2013 e. In the “Equation” window, type in: delta("pH")/delta("Actual volume"). You can also select “delta” from the “Functions” pull-down menu, and the “pH” and “Actual volume” column names from the “Variables” menu. f. Now, change your vertical axis from “pH” to “Derivative” by clicking on the title for the y-axis. The peak on the curve should correspond to the volume at the equivalence point. To show this go to >> Analyze >> Statistics. Then select the derivative curve. This will show the Peak value and other numbers in a Statistics Box. Be sure to include this in your graphs. g. Before you finish up, show your Results to the RE, TA or a mentor to verify that things look right and that you understand what is going on. h. Print out a derivative plot. Then, in the “File” menu, use “Export” to save the data as tab delimited .TXT file for further analysis. i. Save your .TXT file and your Experiment File (.cmbl) on your flash drive, UT Webspace, or email it to yourself so that you have a copy. 9) Repeat the titration two more times to obtain a total of 3 data sets switching roles with your partner for who does the pipette and who is on the computer. Pour 50ml of Tris buffer into the beaker for the repeat titration. You should also fill the burette with more 0.2M NaOH before each titration. The amount does not need to be exact but you want enough to do a run – so about 45-50 ml. It is best to keep all your data on the same graph/sheet so that they aren’t separate figures in the report. (i.e. you will have 1 pH graph and 1 Derviative of pH graph, each with 3 curves) initial burette reading (Vinitial) for Titration #2 starting pH of your 0.1M Tris buffer? initial burette reading (Vinitial) for Titration #3 starting pH of your 0.1M Tris buffer? _______ ml _____________ Titration #2 _______ ml _____________ Titration #3 Analyze these last 2 runs. For each of your Titrations – be sure your graphs are labeled. To show this go to >> Analyze >> Statistics. Then select the curve. This will show numbers and the name of the curve in a Statistics Box to label your graphs. Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 6 Virtual Drug Screening stream Spring 2013 Save your Experiment File (.cmbl) on your flash drive, UT Webspace, or email it to yourself so that you have a copy. Make sure you and your partner both have a copy of the data. Save all of your Raw Data (i.e. CMBL files) online (e.g. UT Webspace) because you might need to go back and analyze/change something. Cleanup Please disassemble the LabPro pH system. Place it back in the box it originated from and return it to the drawer from which it was retrieved. Rinse the probe with diH2O using a squirt bottle re-place it in the storage solution. Then place it back in the cabinet – NOTE: place these upright so that they don’t leak Pour out the 0.2M NaOH remaining in the burette into the basic waste container in the hood. Wash all glassware used. Rinse out the burettes well and allow the water to drain through the stopcock several times to prevent residue from collecting inside. Open the stopcock and place the burette back in the drawer from which it was retrieved. Clean up the area of the Lab bench that you have been using. The stir plates may stay on the bench. Return the metal post, base and clamps to their respective places. Clean the bench surface with a paper towel. Be considerate and return it in a neat and clean status since this is a shared lab. Sign out with mentor. Analysis Be sure to address these points in your analysis section as well as any other information that should go there. 1) Please include all three titration curves and derivative plots in your Lab report (ideally 3 curves per plot for the 3 trials) 2) What is the pH at the equivalence point for each of your titration curves? Show the Average and Standard Deviation too. 3) What is the pH at half the equivalence point for each of your titration curves? Show the Average and Standard Deviation too. 4) Based on all your data, what is the average pKa of Tris? Please calculate the standard deviation as well. Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 7 Virtual Drug Screening stream Spring 2013 5) To test out your empirical findings, using the Henderson-Hasselbalch equation and the pKa you derived for Tris: a. Calculate the pH value of your buffer if you were to have added 15ml of 0.2M NaOH solution to 50ml of 0.1M Tris buffer. You need to know the starting pH of your 0.1M Tris solution (pH before you started titrating with NaOH). b. Does the pH value you calculate from this hypothetical amount in step ‘a)’ correspond with your experimental findings – i.e. the pH reading when you had gotten to 15 ml on your graph? If not, why? What are the sources of error in this experiment? HA H A products H A HA reactants since, pKa -logKa Ka equilibriu m constant H A pKa log HA A pKa -log H log HA since, pH -log H A - - Henderson Hasselbalc h Equation pH pKa log HA 6) Are your results good? How do you determine the precision and accuracy of your experimental results? 7) Can you suggest different variations to this experiment that can be tried in the future? Hint: what are the variables in this experiment? Could you add others? Conclusions: summation statement of what was done in the lab. State key finding(s). Then address future directions and/or applications of how this fits in the big picture of what VDS does. Adapted from Dr. Vivian Feng’s Lab 4- Unknown acid Lab handout 8 Virtual Drug Screening stream Spring 2013 How to Measure pH with LoggerPro Materials: Vernier pH probe, LabPro interface, Computer with Logger Pro, pH buffers of pH 4, pH 7 and pH 10, waster beaker, squeeze bottle (or use disposable plastic pipette bulbs) of deionized water (Nanopure) If the pH electrode has been stored for more than 24 hours it will be in a storage solution (prepared by dissolving 10.0 g KCl in 100 mL of standard pH 4 buffer). Unscrew the cap on the storage bottle before removing the electrode from the cap and the rubber gasket. Place the storage bottle in a place where it will not be tipped over while using the pH electrode. Rinse the electrode with distilled water making sure to rinse off the end and outer body. Be careful not to touch or rub the glass bulb at the electrode's end. Prior to performing measurements, the pH electrode needs to be calibrated. Click on the "pH = …" in the status window in the upper left-hand corner of the window. This will bring up the sensor configuration window shown in Fig. 1. Click on the pH electrode on channel 1 and select “Calibrate” from the menu that pops up. Figure 1. Logger Pro sensor configuration window The sensor setting window, shown in Fig. 2a, will then be displayed. Live Calibration is the default setting. We will be calibrating the electrode using a standard two-point calibration which requires us to immerse the pH electrode into two buffer solutions of known pH. To calibrate the electrode click “Calibrate Now”. 9 Virtual Drug Screening stream Spring 2013 Once you have clicked on the Calibrate Now button, the window will update to that shown in Fig. 2b. Next, immerse the pH electrode in a standard pH 7 buffer, and wait until the “Channel Input:” box is reading a stable signal (measured in units of volts). A stable signal is signified by potential changes by less than ±0.01 V in 30 sec. At this point enter the value 7.00 in the “Enter Value:” box and click the Keep button. Figure 2. Logger Pro sensor settings window Once the first buffer's pH has been entered, remove the electrode from the buffer and rinse it copiously with distilled water into a labeled waste beaker. Next immerse the electrode in the second standard, either a pH 4, OR a pH 10 buffer depending on the expected pH of the solution you will be measuring. Wait for the reading to stabilize and then enter the value 4.00 or 10.00 in the “Enter Value:” box under Reading 2. Click the Keep button, and then the Done button. The electrode is now calibrated and ready to measure the pH of other solutions. Before transferring the electrode to a new solution, rinse the electrode copiously with distilled water. When you are finished making measurements, the pH electrode may be stored in the capped storage bottle with storage solution. 10 Virtual Drug Screening stream Spring 2013 Burettes and Titration A burette (also spelled buret) is used to dispense known amounts of a liquid reagent in experiments for which such precision is necessary, such as a titration experiment. Burettes are extremely precise: class A burettes are accurate to ±0.01 mL. Before you start using a burette, it needs to be properly prepared. Follow the procedure outlined below to prepare a burette: 1. Close the stopcock, and run some DI water using a DI squirt bottle into the top of the burette. Tilt the burette at an angle with a rocking motion to let the water rinse all of the inside of the burette. 2. Open the stopcock, and drain out the water from the tip into the drain. 3. Repeat the above steps at least twice. 4. Close the stopcock and (using a funnel, if you prefer) pour some of the titrant in from the top of the burette (no more than ¼ of the total burette volume). Remove the funnel. 5. Tilt the burette at an angle to rinse all the inside of the burette with titrant solution. 6. Place the burette into the clamp (DO NOT tighten the clamp too much. It may crack the burette). Make sure the burette is perfectly vertical and stable. 7. Drain the titrant into a waste beaker. Label the temporary waste beaker. 8. Fill the burette with the titrant to the top (~50 ml). Do NOT try to aim at the 0.00 ml mark. Just get it near the top within the marked area. Remove the funnel. 9. Place the waste beaker underneath the burette tip, and open the stopcock to let the titrant run down to fill the burette tip. Make sure air bubbles aren’t trapped around the stopcock. If air bubbles are present during a titration, volume readings may be in error. If you have trouble getting air bubbles out, call your instructor or TA for help. 10. Rinse the tip of the burette with water from a wash bottle and remove any drops adhering to the burette tip by touching on the side with a clean Kimwipe. After a minute, check for solution on the tip to see if your burette is leaking. The tip should be clean and dry before you take an initial volume reading. A few tips for using and reading a burette: A 50 mL burette can be read to ±0.01 mL by extrapolating the last decimal place. Make sure you record enough significant figures in your readings. For example: “5.2 mL” is not the same as “5.20 mL”. This will affect the number of significant figures you report at the end. Read the bottom of the meniscus. Be sure your eye is at the level of meniscus, not above or below. Reading from an angle, rather than straight on, results in a parallax error. Keep in mind that you are interested in the Volume Change (Vfinal-Vinitial) in a titration. Therefore, you don’t necessarily need to refill the burette to the top to start a titration. 11