Microsoft Word
advertisement
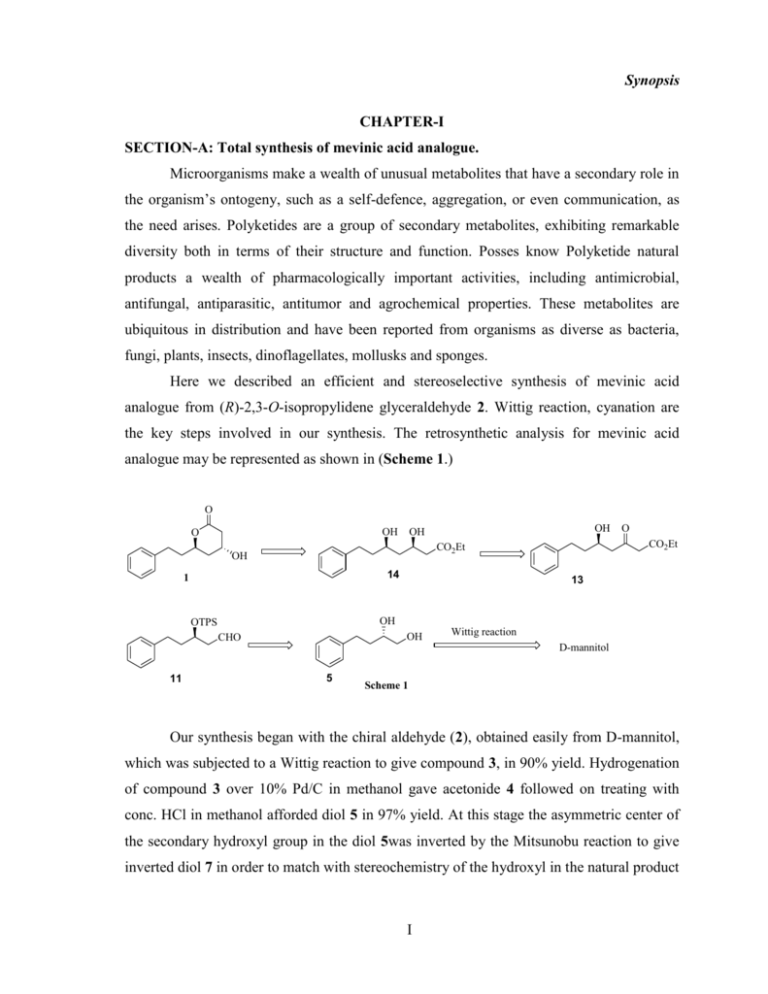
Synopsis CHAPTER-I SECTION-A: Total synthesis of mevinic acid analogue. Microorganisms make a wealth of unusual metabolites that have a secondary role in the organism’s ontogeny, such as a self-defence, aggregation, or even communication, as the need arises. Polyketides are a group of secondary metabolites, exhibiting remarkable diversity both in terms of their structure and function. Posses know Polyketide natural products a wealth of pharmacologically important activities, including antimicrobial, antifungal, antiparasitic, antitumor and agrochemical properties. These metabolites are ubiquitous in distribution and have been reported from organisms as diverse as bacteria, fungi, plants, insects, dinoflagellates, mollusks and sponges. Here we described an efficient and stereoselective synthesis of mevinic acid analogue from (R)-2,3-O-isopropylidene glyceraldehyde 2. Wittig reaction, cyanation are the key steps involved in our synthesis. The retrosynthetic analysis for mevinic acid analogue may be represented as shown in (Scheme 1.) O OH O OH OH OH 14 1 O CO2Et CO2Et 13 OH OTPS OH CHO Wittig reaction D-mannitol 11 5 Scheme 1 Our synthesis began with the chiral aldehyde (2), obtained easily from D-mannitol, which was subjected to a Wittig reaction to give compound 3, in 90% yield. Hydrogenation of compound 3 over 10% Pd/C in methanol gave acetonide 4 followed on treating with conc. HCl in methanol afforded diol 5 in 97% yield. At this stage the asymmetric center of the secondary hydroxyl group in the diol 5was inverted by the Mitsunobu reaction to give inverted diol 7 in order to match with stereochemistry of the hydroxyl in the natural product I Synopsis 1 at C-2′. The primary hydroxyl group in 7 was protected with tosylchloride using triethylamine and catalytic amount of Bu2SnO to produce tosylated compound 8 in 79% yield with the minor tosylation at the secondary hydroxyl group of 8. The tosylate 8 was converted to corresponding nitrile 9 in 91% yield by the reaction of KCN in ethanol and H2O (3:2) at room temperature. The secondary hydroxyl group in the nitrile 9 was protected with TBDMSCl/imidazole to give tertiary-butyldimethylsilyl ether 10 in 98% yield. The nitrile function was reduced with DIBAL-H in dry dichloromethane at -78 0C to afford the aldehyde 11 in 70% yield (Scheme 2). O O O O [Ph(CH2)3Ph3P]Br O MeOH, rt, 6h, 96% nBuLi, THF 0 0C, 0.5h, 90% H O H2, 10%Pd/C O 2 4 3 PPh3, DIAD OH OR OH NO2C6H4CO2H 2M HCl, MeOH OR THF, rt 2h, 90% rt, 1h, 97% 5 Na, MeOH rt, 1h, 94% R = NO2C6H4CO 6 OH OH OH TsCl, (C2H5)3N (nBu)2SnO, 0 0C-rt 7 TBDMS-Cl, Imidazole dry CH2Cl2 rt, 3h, 98% OH OTs 4h, 79% 8 OTPS CN DIBAL-H, dry CH2Cl2 CN KCN Ethanol/H2O (3:2) rt, 12h, 91% SPTO 9 O H 0 10 -78 C, 0.5h, 70% 11 Scheme 2 TBS protected aldehyde in compound 11 was reacted with ethyl diazoacetate and tin chloride ( SnCl4) in the presence of dichloro methane at 0 0C to give the corresponding β-keto ester 12. Deprotection of TPS group of compound 12 was done by treatment with 0.1 equivalent of PPTS in MeOH to afford compound 13 in 90% yield. In order to get syn 1,3-diol, compound `13 was subjected to syn-selective reduction using 2 equivalent of catecholborane as reducing agent in dry THF at –10oC, which afforded the desired syn diol II Synopsis 14 in 95% yield. Protection of 1, 3 hydroxy groups of compound 14 was effected in dry dichloro methane with 1.5 equivalent of 2, 2-DMP in the presence of catalytic amount of pyridinium toluene 4-sulphonicacid for 3 h at room temperature to furnish 15 in 87% yield. The cyclization of 15 was effected in dry DCM in the presence of a catalytic amount of Para toluene-4-sulphonic acid for 6h at room temperature afforded final compound 1 in 84% yield (Scheme-3). SPTO OTPSO O H O ethyl diazoacetate, SnCl4 OEt TBAF, MeOH 4Ao MS poeder, OoC-rt 11 OH 12 O OH O OH Catecholborane O OEt OEt 2,2-DMP, PPTS DCM, O0 C-rt THF, -100 C 14 13 O O O O O OEt 2,2-DMP, PPTS DCM, O0 C-rt OH 1 15 Scheme-3 SECTION-B: Total synthesis of Goniothalamin. During the course of a program aimed at the synthesis of bioactive molecules, we selected goniothalamin 16, a naturally occurring styryl lactone was isolated in 1967 from the dried bark of Cryptocarya caloneura. Later it was isolated from Cryptocarya moschata, Bryonopsis laciniosa and various species of Goniothalamus. Gonionthalamin 16 has shown invitro cytotoxic effects especially by inducing apoptosis on different cancer cell lines [cervical carcinoma (Hela), gastric carcinoma (HGC-27), breast carcinoma (MCF-7, T47D, and MDA-MB-231), leukemia carcinoma (HL-60), and ovarian carcinoma (Caov-3)]. Goniothalamin 16 is a potent mosquito larvicide and also shows weak antibacterial and III Synopsis significant antifungal activity against a wide range of gram positive and gram-negative bacteria and fungi. During the course of a program aimed at the synthesis of bioactive molecules, we selected goniothalamin 16; an antitumor natural product was selected as our synthetic target. Here we reported an efficient and stereoselective synthesis of goniothalamin 1 from (R)-glycidol. Opening of epoxide with ethyl propiolate, cis hydrogenation and JuliaKociensky olefination are the key steps involved in our synthesis. The retro synthetic approach for (R)-goniothalamin 16 may be represented as shown in Scheme 4. O O O OH O MPMO OEt O HO HO O 16 20 18 17 Scheme 4 The synthesis of goniothalamin 16 is based on a sequence of reactions starting from commercially available (R)-glycidol 17, which was protected with p-methoxy benzyl bromide in presence of NaH in THF yielding the corresponding epoxy ether 18 in 95% yield. 18.The opening of epoxy ether 18 with ethylpropiolate in presence of BuLi in THF at –78 0C to produce the secondary alcohol 19 in 86% yield. The secondary alcohol 19 was hydrogenated using Lindlar’s catalyst in the presence of hydrogen atmosphere in benzene to the (Z)-α,β-unsaturated ester 20 in 91% yield.. Compound 20 was subjected to lactonisation by treating the cis ester 20 in chloroform and using p-toluene sulfonic acid to afford the compound 21 in 92% yield. The deprotection of MPM group in compound 21was proceeded smoothly with DDQ in dichloromethane/H2O (8:2) yielding the alcohol 22 in 92% yield. The alcohol 22 was subjected to Swern oxidation to yield the unstable aldehyde in 93% yield, which was directly used in Wittig reaction with benzyl triphenyl phosphonium bromide in the presence of n-BuLi or KtObu provided 16 with the poor E/Z IV Synopsis (1:4) selectivity. Attempts to employ the Julia-Kociensky protocol to selectively install the desired E double bond configuration provided only goniothalmin 16 in 30% yield. O HO NaH, THF, 0 0C-r.t. O MPMO Ethyl propiolate, n-BuLi, BF3.Et2O 3h, 95% OH MPMO OEt THF, -78 0C, 30 min. 90% 17 18 19 O O Pd/CaCO3, benzene OH MPMO OEt H2, 1h, 91% PPTS, CHCl3 1h, r.t., 94% 20 O O DDQ CH2Cl2/H2O (8:2) MPMO 4h, 92% O O 21 O (i) (COCl)2, CH2Cl2 DMSO, Et3N, -78 0C, 30 min HO (ii) then a solution of the 23, KHMDS, THF, -78 0C (< 30%). 22 Ph N N N N S O O R1 O R2 (R)-(Z)-(16), R1 = Ph and R2 = H (R)-Goniothalamin (16), R1 = H and R2 = Ph Ph 23 Scheme-5 CHAPTER-II SECTION-A: The stereo selective synthesis of tiruchanduramine (24). Synthesis of natural products is a challenging area. The total synthesis of natural products involves prudent way of preparing and joining/stitching the various segments of the active molecule using the different existing methods. Generally, the boundaries/targets are defined by natural products, which at present is far reaching in spite of several advancements in synthetic methodologies have been achieved. A novel -carboline guanidine alkaloid tiruchanduramine 24 isolated from an ascidian Synoicum macroglossum, which was collected at Tiruchandur, Tamilnadu, India during February 2002. Tiruchanduramine 24 showed promising α-glucosidase inhibitory activity (IC50 78.2 lµg/mL) as compared with acarbose16 at 100 lµg/mL as the standard. The plausible retro-synthetic analysis of the title compound is shown in the Scheme-6, which recognized two conceivable segments 1) aromatic and 2) aliphatic part of V Synopsis the molecule. The aromatic part of the molecule 3- carbolinic acid (29) can be readily synthesized from the amino acid tryptophan(25). H N O N N H NH N H OH O N H N H N H N OH NH + H2N NH2 40 24 O O O HCHO OR + OH + NH2 N H N H 25 Tryptophan Scheme-6 O H2N N 37 R = H, OMe 29 Aromatic ring synthesis: The 3--carbolinic acid (29) was prepared using Pictet-Spengler conditions. Tryptophan 25 was reacted with aqueous formaldehyde in the presence of H2SO4 to give tetrahydro 3--carbolinic acid (26) in 78% yield. Compound 26 was esterfied by bubbling dry HCl gas in dry MeOH to give methyl ester 27 in 85% yield. Compound 27 was aromatized using Pb(OAc)4 in acetic acid in the presence of oxalic acid to give 3-carboline methyl ester (28) in 56% yield. Compound 28 was further hydrolyzed with NaOH in EtOH to give corresponding 3--carbolinic acid (29) in 90% yield (Scheme-6). O O OH NH2 N H 31 Tryptophan HCHO, H3O+ 78% O OH MeOH, HCl NH N H 85% N H 32 33 O Pb (OAC)4 Ac OH, 56% OMe O OMe N N H 34 b-Carboline methyl ester NaOH OH 90% N H 35 Scheme-4 VI N b-Carbolinic Acid N Synopsis 1) Preparation of Aliphatic Fragment: In order to prepare aliphatic part containing guanidine segment (37) of tiruchanduramine, compound 37 was prepared starting from commercially available Lmalicacid (30). L-malicacid (30) was treated with methanol and catalytic amount of H2SO4 at reflux conditions for 12h to furnish the corresponding dimethyl ester (31) in 90% yield. Reduction of this dimethyl ester with 1.2 equivalents of BH3.DMS and catalytic amount of sodium borohydride in dry THF at room temparature for 3h afforded the corresponding diol (32) in 80% yield. Protection of 1,2 hydroxy groups of compound 32 was effected in dry DCM with 1.2equivalent of 2,2-dimethoxy propane (2,2-DMP) in the presence of catalytic amount of p-toluene sulfonic acid (PTSA) for 3 h at room temperature to furnish 33 in 85% yield. The ester group in 33 was reduced to alcohol 34 Using LiAlH4 at oC-rt in 90% yield. The primary hydroxyl group in compound 34 as tosylated by using tosylchloride and triethylamine in dichloromethane at room temperature to provide the tosylate 35 76% yield Compound 35 s treated with 1.2 equivalents sodium azide in dry DMF to afford the azide compound 36 in 80% yield. The azide compound 36 as hydrogenated by using 10% Pd/C and NaHCO3 in ethyl acetate under hydrogen atmosphere at room temperature to afford the acetonide protected amine compound 37 in 90% yield (scheme-7). OH O HO MeOH, Cat. H2SO4 OH O OH OMe O O PTSA O O THF, 0 oC-rt, 3h 33 O O NaN3 OTs 35 DMF, 70 oC, 3h THF, 0 oC-rt OMe 32 O OMe O HO 31 LiAiH4 O OH BH3.DMS reflux, 90% 30 2,2-DMP, DCM O MeO TsCl, (C2H5)3N 0 0C-rt, 4h, 85% OH 34 O O O N3 36 Scheme-7 VII O NH2 37 Synopsis The amine 37 and 3--carbolinic acid (29) were coupled using DCC/DMAP in dry DCM to give corresponding amide 38 in 70% yield. Also in a different experiment amine 37 and 3-carbolinic acid (29) were coupled using HOBT/EDCI to give amide 38 in 68% yield. Acetinide protected amide compound (38) was reacted with BOC anhydride and triethylamine in the presence of dichloromethane afforded the corresponding N-BOC protected amide (39) in 85% yield. The deprotection of acetonide group in compound 39 was achieved by using pyridinium p-toluene sulfonate (PPTS) in dry methanol at room temperature to afford the corresponding Boc protected diol 40 in 95% yield. Here PPTS was working as a very mild catalyst, which does not deprotect the BOC and deprotects only acetonide group. A careful literature survey revealed that Murray Goodman et al reported new guanidinylation reagents in the year of 1998. These reagents consist of N, N', N''-tri-Bocguanidine (a) and N, N', N''-tri-Cbz-guanidine (b), which allows a facile conversion of alcohols to substituted guanidine’s. They synthesized a series of arginine analogues by reacting of primary and secondary alcohols with guanidinylation reagents a and b under Mitsunobu conditions. And reported N, N'-di-Boc-N''-trifylguanidine (c), and N, N'-di-CbzN''-trifylguanidine (d). The trifyldiurethane-protected guanidine c was utilized to guanidinylate primary and secondary amines under mild conditions with high yield in solution and on solid phase. NBoc BocHN NCbz NHBoc CbzHN NHCbz a b NTf NTf BocHN NHBoc CbzHN c NHCbz d At this juncture again we went back to diol 40 and reacted with tri BOC guanidine (a) under Mitsunobu conditions to afford the tetra Boc protected tiruchanduramine 41.The VIII Synopsis Boc protected groups from the target molecule 41 was removed, which on treatment with 0.5 N HCl in dry methanol gave tiruchanduramine hydrochloride (24). O O O OH 37 N H 29 N O HOBT+ EDCI DIPEA, DMF 0 oC-rt, 6h 38 PPTS MeOH, rt, 12h OH OH TPP, DEAD, THF N H Tri Boc Guanidine 40 Boc N O N Boc N N Boc 39 N Et3N, DCM 0 oC-rt, 2h O N H N N N H (BOC)2O N H O O N Boc O NH N H N Boc H N O TPP, DEAD, THF Tri Boc Guanidine 41 Scheme-8 IX N N H 24 NH N H N H Synopsis CHAPTER-II SECTION-B: Chemical modification of polybrominated diphenylethers. Br OH O Br Br O K2CO3, Acetone Reflux, 6h Br OR Br Br Br Br Scheme-1 R = Me R = Ac R = Allyl R = - Allyl R = (CH2)n-OH n = 2, 3, 4, 5, 6 R = -CH(CH2)- COOEt R = - CH2- Ph R = CH2- CO-Ph O R= - H2C Cl X Synopsis Br OH O Br Br Br K2CO3, Acetone O 1, 5-Dibromopentane Br Reflux, 4-5h Br Br O O K2CO3, CH3CN Br Br Reflux, 6h Br R O R Br Br Br R = Morpholine R = N-methyl piperzine R = N-Boc piperzine R = Homo Boc piperzine The above all the compounds showed antibacterial activity against gram positive and gramnegative bacteria. CHAPTER III XI Synopsis Synthetic organic chemistry has always been a frontier area of research due to its impact on the material and biological sciences. The scientific community in this area is constantly involved in developing efficient methodologies, novel reactions and processes that will lead to the synthesis of desired target molecules and their derivatives with ease. Synthetic organic chemistry is not only a tool for obtaining compounds that can be utilized for understanding biological functions or the behavior of materials, but it also leads to the creation of novel drug or drug like candidates and of novel materials with interesting properties. 1) The chemo selective tetrahydropyranylation of primary alcohols using La(NO3)3.6H2O as a catalyst: The tetrahydropyranyl (THP) group is frequently used for the protection of alcohols and phenols due to their ease of preparation and stability under a wide variety of reaction conditions such as with hydrides, alkylating reagents, Griganard reagents and organometallic reagents In addition, they also serve as stable protecting group in peptide, nucleoside and nucleotide, carbohydrate and steroid chemistry. Tetrahydropyranylation is a general and important protecting group to protect hydroxy groups in multistep organic transformations. There are several reagents available for tetrahydropyranylation of alcohols, which include the use of protic and Lewis acids. However several reported methods are associated with certain drawbacks, which include long refluxing reaction time conditions and the use for other functionalities. Thus there is a need for suitable, mild and selective efficient alternative for the protection of hydroxyl functionality as THP ether. As a part of our ongoing exploration of the acidic catalytic activity of lanthanum (III) nitrate hexahydrate, we envisaged the chemo selective protection of primary alcohols with THP ethers in the presence of secondary alcohols using La(NO3)3.6 H2O (Scheme-11). HO THPO O HO BnO O O La(NO3)3.6H2O O HO O DHP, rt, 2-3 h BnO Scheme 1 XII O Synopsis Encouraged by the success of the reaction, various benzylic and allylic alcohols were subjected to the tetrahydropyranylation with excellent yields. It has been observed that the substrates containing other acid labile functional groups such as actinide, TBDMS, isopropylidine protected diols were intact during tetrahydropyranylation . In order check the versatility of the reagent La (NO3)3.6H2O, a mixture of 2-phenyl ethanol and 1-phenyl ethanol were reacted with dihydropyran in the presence of lanthanum (III) nitrate hexahydrate to give exclusively THP protected 2-phenyl ethanol (Scheme-12). OH OH OH La(NO3)3.6H2O + OTHP + DHP, rt, 2-3 h Scheme-2 In conclusion, we have developed a simple, efficient, mild, and highly selective process for tetrahydropyranylation of alcohols using Lanthanum nitrate as a catalyst. The mild, environmentally clean reaction conditions, and less reaction times with high yields the advantages of the present methodology. 2) Acetylation of alcohols, phenols and amines with acetic anhydride using La(NO3)3.6H2O as a catalyst: Functional group protection strategies are central to target molecule synthesis. The protection of alcohols, phenols and amines are fundamental and useful transformations in organic synthesis. Among the many protecting groups for hydroxyls, phenols and amines, acetate is used with high frequency. Although, numerous methods are available for the preparation of acetates using acetic acid and a protic acid, acetic anhydride and pyridine are the most commonly used reagents. 4-(Dimethylamino)pyridine (DMAP) and 4pyrrolidinopyridine (PPY) catalyze the acetylation of alcohols. However, most of these reported methods suffer from one or more disadvantages like long reaction times, harsh reaction conditions, the occurrence of side reactions, toxic reagents, poor yields of the desired products and intolerance of other functional groups. Here, we report a mild and XIII Synopsis efficient method for the acetylation of alcohols, phenols and amines using acetic anhydride in the presence of La(NO3)36H2O (Scheme 13). THPO THPO O HO BnO O O La(NO3)3.6H2O O AcO Ac2O, rt, 10-20 min BnO O O Scheme-1 We found that La(NO3)36H2O is an efficient and mild acidic catalyst for the acetylation of alcohols with Ac2O under solvent-free conditions. In order to establish the catalytic activity of La(NO3)36H2O, we carried out the acetylation of glucose diacetonide (1 mmol) with acetic anhydride (1.2 mmol) using La(NO3)36H2O (5 mol %) at room temperature which gave the corresponding acetate in 96% yield. Encouraged by the success of this reaction, various primary, secondary, benzylic and allylic alcohols and phenols) and amines were subjected to acetylation in excellent yields. Substrates containing other acid labile functional groups such as acetonide, TBDMS and isopropylidene protected diols remained intact during acetylation. Interestingly, when diols were subjected to acetylation, monoacetates were formed as the major products with very good yields. From these results it is evident that acetic anhydride and lanthanum (III) nitrate hexahydrate is an excellent combination for the acetylation of alcohols, phenols and amines under solvent- free conditions. Thiols and thiophenols could not be acetylated using this method. 3) Synthesis of α-amino nitriles using Lanthanum (III) nitrate hexahydrate or Gadolinium (III) chloride hexahydrate as a catalyst: The addition of cyanide anion to imines (the Strecker reaction) provides one of the most important and straight forward method for the synthesis of α-aminonitriles, which are useful intermediates for the synthesis of aminoacids and nitrogen containing heterocycles such as thiadiazoles and imidazoles, etc. The classical Strecker reaction usually carried out in aqueous solution and the work-up procedure is also tedious. Thus several modifications of Strecker reaction have been reported using a variety of cyanide reagents, such as diethyl phosphorocyanidate and α- XIV Synopsis aminonitriles, as well as catalysts, such as InCl3, BiCl3, Montmorillonite KSF clay, Sc(OTf)3, Bromodimethyl sulfonium bromide under various reaction conditions. The use of trimethylsilyl cyanide is a safer and more effective cyanide anion source for the nucleophillic addition reactions of imines under mild conditions. However, many of these methods involve the use of expensive reagents, harsh conditions, extended reaction times, and also require tedious work-up leading to the generation of a large amount of toxic waste. Further more many of these catalysts are deactivated or sometimes decomposed by amines and water that exist during imine formation. In order to overcome these problems recently one-pot procedures have been developed for this transformation. In continuation of our work to develop new organic transformations, here we report that a mild efficient and environmentally benign catalysts, for the preparation of α-aminonitriles from carbonyl compounds, amines and trimethylsilyl cyanide in presence of La(NO3)3.6H2O or GdCl3.6H2O in acetonitrile at room temperature (Scheme 14). R-CHO + R'-NH2 + Me3SiCN 1 2 La(NO3)3.6H2O or GdCl3.6H2O MeCN, r.t. 1.0 - 3.0 h 3 NHR' R CN 70 -98% 4 Scheme-1 In conclusion that the present procedure using Lanthanum(III) nitrate hexahydrate or Gadolinium(III) chloride hexahydrate provides an efficient synthesis of α-aminonitriles by a onepot three component coupling of carbonyl compound, amine and TMSCN. The salient features of this methodology are: General applicability to different types of aldehydes and amines, using cheap and commercially available reagents, short reaction times, and high yields of products. Finally this report provides us towards environmentally-friendly chemical processes. 4) Synthesis of homoallylic alcohols using Lanthanum (III) nitrate hexahydrate as a catalyst: The Lewis acid catalyzed addition of allylstananes to aldehydes for the preparation of homoallylic alcohols is important organic tranceformation as they are important building XV Synopsis blocks and versatile intermediates for the synthesis of natural products . This has led to development of new synthetic methodologies for the synthesis of homoallylic alcohols. Organic reactions using mild and water tolerant catalyst have been received much attention in recent years as they can conveniently be handled and removed from the reaction mixture, making the experimental procedure simple and eco-friendly. In the course of study in above transformations. O R H + OH La(NO3)3.6H2O SnU3B CH3CN, r.t R Scheme-1 In this report we have described an efficient method for the synthesis of homoallylic alcohols using mild Lewis acid catalyst. The reaction proceeds efficiently and smoothly at room temperature and the products are obtained in excellent yields. Furthermore, the reaction conditions are very mild, no by-products were observed. Initially, we have reacted allyltribultylstannane with benzaldehyde in acetonitrile at room temperature using catalytic amount of lanthanum (III) nitrate hexahydrate (5 mol%) to give corresponding homoallylic alcohol in 99% . In order to check the veracity of the catalyst we paid attention to other substituted aromatic aldehydes, ,-unsaturated and aliphatic aldehydes to give corresponding homoallylic alcohols. In all the cases the obtained yields are good to excellent. The chiral substrates containing acid labile protective group such as acetonideprotected diols were also converted into corresponding products without disturbance of chirality as well as acetonide. In conclusion we described mild and an efficient synthesis of homoallylic alcohols by the reaction of aldehydes with allyltribultylstannane using catalytic amount of La(NO3)3.6H2O. The present method has the advantage of mild reaction conditions, comparatively less reaction time and improved yields of the products. We thought that it is an important addition of existing methodologies. XVI









