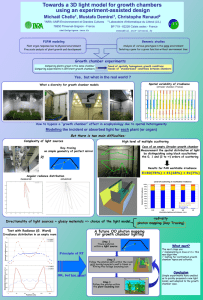
Table of Contents - University of Colorado at Boulder
advertisement

Microbial Detection Arrays Elizabeth Newton Project Manager Shayla Stewart Systems Engineer Ted Schumacher Lead Thermal Engineer/Assistant Project Manager Jeff Childers Lead Structural Engineer Charles Vaughan Lead Electrical Engineer/Chief Safety Officer Dave Miller Lead Fabrication Engineer Steven To Chief Financial Officer Sameera Wijesinghe Webmaster AES – University of Colorado, Boulder ASEN 4018 – Senior Projects I: Design Synthesis Fall Final Report December 18, 2006 Advisors: Dr. James Maslanik and Dr. Jeffrey Forbes Customers: BioServe and Tufts University Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table of Contents List of Figures ........................................................................................................................... 5 List of Tables ............................................................................................................................ 7 List of Acronyms ...................................................................................................................... 8 1.0 Project Overview and Requirements .......................................................................... 11 1.1.0 Objective ............................................................................................................. 12 1.2.0 Instrument Operation .......................................................................................... 12 1.3.0 Mars/Earth Comparison ...................................................................................... 13 1.4.0 Design Requirements .......................................................................................... 14 1.4.1.0 Requirement Importance ................................................................................. 16 1.5.0 Deliverables ........................................................................................................ 19 2.0 System Architecture .................................................................................................... 20 2.1.0 Critical Components ........................................................................................... 22 2.1.1.0 Autoclaves....................................................................................................... 22 2.1.2.0 Peristaltic Pumps............................................................................................. 22 2.1.3.0 Reagent Water ................................................................................................. 23 2.1.4.0 Valve ............................................................................................................... 23 2.1.5.0 Test Chambers ................................................................................................ 23 2.1.6.0 Mixers ............................................................................................................. 23 2.1.7.0 Tubing ............................................................................................................. 23 2.1.8.0 Motors ............................................................................................................. 23 2.2.0 Experiment Timeline .......................................................................................... 24 2.3.0 Electrical Subsystem ........................................................................................... 24 3.0 Development and Assessment of System Design Alternatives .................................. 26 3.1.0 System Architecture Pros and Cons .................................................................... 27 3.2.0 System Architecture Options .............................................................................. 29 3.2.1.0 Option A – Monobox ...................................................................................... 29 3.2.2.0 Option B – Single Sterilization, Shared Environment .................................... 30 3.2.3.0 Option C – Single Sterilization, Separate Environment ................................. 31 3.2.4.0 Option D – Dual Sterilization, Separate Environment.................................... 32 3.2.5.0 Option E – Dual Sterilization, Shared Environment....................................... 33 3.3.0 Quantitative Analysis .......................................................................................... 34 3.4.0 Selected System Architecture ............................................................................. 35 4.0 System Design-To Specifications ............................................................................... 36 4.1.0 Reaction Sample Handling ................................................................................. 37 4.1.1.0 Environmental Chamber ................................................................................. 37 4.1.2.0 Support Structure ............................................................................................ 37 4.2.0 Reaction Sample Delivery .................................................................................. 38 4.2.1.0 Clean Room Assembly ................................................................................... 38 4.3.0 Nominal and Peak Power Consumption ............................................................. 38 4.3.1.0 System Insulation ............................................................................................ 38 4.4.0 Unit Disassembly ................................................................................................ 38 4.5.0 Operational Environment .................................................................................... 39 4.5.1.0 Instrument Mass and Volume ......................................................................... 39 Page 1 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.5.2.0 Instrument Cost ............................................................................................... 39 4.5.3.0 Component Replacement ................................................................................ 39 4.5.4.0 Instrument Reliability ..................................................................................... 39 4.5.5.0 Field Environment .......................................................................................... 39 5.0 Development and Assessment of Subsystem Design Alternatives ............................. 40 5.1.0 Reaction Chamber ............................................................................................... 41 5.1.1.0 Subsystem Design to Requirements................................................................ 41 5.1.2.0 Requirement Analysis ..................................................................................... 41 5.1.3.0 Internal Volume .............................................................................................. 42 5.1.4.0 Material Requirements .................................................................................... 42 5.1.5.0 Heating Element Trade study.......................................................................... 44 5.1.6.0 Cooling Element Trade Study......................................................................... 46 5.1.7.0 Insulation Trade Study .................................................................................... 47 5.1.8.0 Mixing Trade study ......................................................................................... 49 5.1.9.0 Temperature Sensor Trade study .................................................................... 50 5.1.10.0 Pressure Sensor Trade study ....................................................................... 51 5.2.0 Autoclave ............................................................................................................ 53 5.2.1.0 Subsystem Design to Requirements................................................................ 53 5.2.2.0 Autoclave Techniques ..................................................................................... 53 5.2.3.0 Thermal Analysis ............................................................................................ 53 5.2.4.0 Insulation......................................................................................................... 55 5.2.5.0 Material Selection ........................................................................................... 55 5.2.6.0 Heating and Cooling Apparatus ...................................................................... 56 5.2.7.0 Sensors ............................................................................................................ 56 5.3.0 Sample Transportation ........................................................................................ 56 5.3.1.0 Requirements .................................................................................................. 56 5.3.2.0 Material Selection ........................................................................................... 57 5.3.3.0 Valve Selection ............................................................................................... 57 6.0 Subsystem Design-To Specifications ......................................................................... 59 6.1.0 Overall System Architecture ............................................................................... 60 6.2.0 Subsystem Design ............................................................................................... 66 6.2.1.0 Chassis Design ................................................................................................ 66 6.2.2.0 Autoclave Design ............................................................................................ 66 6.2.3.0 Reagent Water Delivery Design ..................................................................... 67 6.2.4.0 Environmental Design .................................................................................... 67 6.2.5.0 Data Acquisition Design ................................................................................. 67 6.2.6.0 Mass ................................................................................................................ 67 6.3.0 Power requirements ............................................................................................ 68 6.3.1.0 Requirements .................................................................................................. 68 6.3.2.0 Power .............................................................................................................. 71 6.4.0 Component List ................................................................................................... 72 7.0 Project Feasibility and Risk Assessment .................................................................... 74 7.1.0 Autoclave Prototype............................................................................................ 75 7.2.0 Mixing Prototype ................................................................................................ 79 7.3.0 Sample Transportation Prototype ....................................................................... 80 7.4.0 Risk Assessment ................................................................................................. 81 Page 2 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 8.0 Mechanical Design Elements ...................................................................................... 83 8.1.0 Sterilization Chambers ........................................................................................ 84 8.1.1.0 Volume and Dimensions ................................................................................. 85 8.1.2.0 Material and mass ........................................................................................... 87 8.1.3.0 Thermal analysis ............................................................................................. 87 8.2.0 Peristaltic pumps ................................................................................................. 91 8.3.0 Reagent water chamber ....................................................................................... 91 8.4.0 Valve assembly ................................................................................................... 91 8.5.0 Reaction chambers .............................................................................................. 92 8.5.1.0 Volume and Dimensions ................................................................................. 93 8.5.2.0 Material and mass ........................................................................................... 95 8.5.3.0 Thermal control ............................................................................................... 95 8.6.0 Environmentally controlled enclosure ................................................................ 96 8.6.1.0 Thermal analysis ............................................................................................. 96 8.6.2.0 Time and Power required for heating/cooling ................................................ 97 8.7.0 Mixer motors....................................................................................................... 98 8.8.0 External case ....................................................................................................... 98 9.0 Electrical Design Elements ....................................................................................... 100 9.1.0 Electrical Diagram ............................................................................................ 102 9.2.0 Power Input and Supply .................................................................................... 103 9.3.0 Sensor Diagram ................................................................................................. 104 9.4.0 Signal Conditioning .......................................................................................... 107 9.5.0 Control Diagrams .............................................................................................. 107 9.5.1.0 Strip heater Diagram ..................................................................................... 109 9.5.2.0 Thermostat .................................................................................................... 109 9.5.3.0 LEDs ............................................................................................................. 109 9.5.4.0 TEC Control .................................................................................................. 110 9.5.5.0 TEC (Thermoelectric cooler) ........................................................................ 111 9.5.6.0 Fan................................................................................................................. 112 9.5.7.0 Peristaltic Pump ............................................................................................ 112 9.6.0 Mixer Control.................................................................................................... 113 9.6.1.0 Mixer ............................................................................................................. 115 9.6.2.0 Computer....................................................................................................... 116 9.7.0 DAQ .................................................................................................................. 116 9.7.1.0 Wire diagram ................................................................................................ 119 9.8.0 Powered Items ................................................................................................... 121 10.0 Software Design Elements ........................................................................................ 123 11.0 Integration Plan ......................................................................................................... 130 11.1.0 Instrument Assembly ........................................................................................ 131 11.2.0 Autoclave Functional Test Plan ........................................................................ 132 11.3.0 Reaction Chambers ........................................................................................... 133 11.4.0 Data Acquisition and Control ........................................................................... 133 12.0 Verification and Test Plan ........................................................................................ 135 12.1.0 Design Requirement Verification Plan ............................................................. 136 12.2.0 Component Functional Verification and Test ................................................... 138 12.3.0 Subsystem-Level Verification and Test ............................................................ 138 Page 3 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 12.4.0 System-Level Verification and Test ................................................................. 139 13.0 Project Management Plan ......................................................................................... 140 13.1.0 Organizational Responsibilities ........................................................................ 141 13.2.0 Work Breakdown Structure .............................................................................. 142 13.3.0 Schedule ............................................................................................................ 143 13.4.0 Specialized Facilities and Resources ................................................................ 144 13.5.0 Overall Budget .................................................................................................. 145 14.0 Appendix A: Mechanical Design .............................................................................. 146 14.1.0 Mechanical Drawing Tree................................................................................. 146 14.2.0 Sterilization Chamber Lid ................................................................................. 147 14.3.0 Sterilization Chamber Body.............................................................................. 148 14.4.0 Sterilization Chamber Bottom .......................................................................... 149 14.5.0 Sterilization Chamber Assembly ...................................................................... 150 14.6.0 Reaction Chamber Cap ..................................................................................... 151 14.7.0 Reaction Chamber Base .................................................................................... 152 14.8.0 Reaction Chamber Body ................................................................................... 153 14.9.0 Reaction Chamber Assembly............................................................................ 154 14.10.0 Reagent Water Chamber Body ..................................................................... 155 14.11.0 Water Chamber Cap ...................................................................................... 156 14.12.0 Top Support Shelf ......................................................................................... 157 14.13.0 External Case ................................................................................................ 158 15.0 Appendix B: Electrical Design ................................................................................. 159 15.1.0 Electrical Schematic Tree ................................................................................. 159 15.2.0 Overall Electrical Schematic............................................................................. 160 15.3.0 Electrical block diagram ................................................................................... 161 15.4.0 Power Diagram ................................................................................................. 162 15.5.0 Sensor Diagram ................................................................................................. 162 15.6.0 Control Diagrams .............................................................................................. 163 15.7.0 Wire Diagram.................................................................................................... 165 15.8.0 Switch Board ..................................................................................................... 165 16.0 Appendix C: Software............................................................................................... 166 16.1.0 Software Tree .................................................................................................... 166 16.2.0 Software Prototype............................................................................................ 167 17.0 Appendix D: Data Sheets .......................................................................................... 168 17.1.0 Motor................................................................................................................. 169 17.2.0 TEC ................................................................................................................... 170 17.3.0 Motor Control ................................................................................................... 172 17.4.0 Pressure Sensor ................................................................................................. 176 17.5.0 TEC Controller.................................................................................................. 177 17.6.0 Peristaltic Pump ................................................................................................ 189 17.7.0 Voltage Regulator ............................................................................................. 192 17.8.0 CPU ................................................................................................................... 193 18.0 Appendix E: References ........................................................................................... 195 Page 4 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 List of Figures Figure 1-1NASA Technology Readiness Level Definitions .................................................. 12 Figure 2-1 Overall System Architecture ................................................................................. 21 Figure 2-2 Internal Components ............................................................................................. 22 Figure 3-1 Option A Schematic .............................................................................................. 29 Figure 3-2 Option B Schematic .............................................................................................. 30 Figure 3-3 Option C Schematic .............................................................................................. 31 Figure 3-4 Option D Schematic .............................................................................................. 33 Figure 3-5 Option E Schematic............................................................................................... 34 Figure 3-6 System Architecture Decision Process .................................................................. 35 Figure 5-1 Sonaer S900PIEZO Ultrasonic Processor ............................................................. 49 Figure 5-2 Butterfly Valves .................................................................................................... 57 Figure 5-3 Needle Valve ......................................................................................................... 58 Figure 6-1 Overall System Architecture ................................................................................. 60 Figure 6-2 Top Level Integration Plan.................................................................................... 61 Figure 6-3 Experiment Timeline ............................................................................................. 64 Figure 6-4 Organizational Chart ............................................................................................. 66 Figure 6-5 Mass Analysis ....................................................................................................... 68 Figure 6-6 Power Summary (Full Sterilization Phase) ........................................................... 69 Figure 6-7 Power Summary (Sterilization Phase) - Staggered Autoclaves ............................ 70 Figure 7-1 Autoclave Thermal Analysis ................................................................................. 75 Figure 7-2 Autoclave Prototype Model .................................................................................. 76 Figure 7-3 Autoclave Prototype Setup .................................................................................... 77 Figure 7-4 Autoclave Pressure Results ................................................................................... 77 Figure 7-5 Autoclave Temperature Results ............................................................................ 78 Figure 7-6 Prototype Mixer .................................................................................................... 79 Figure 8-1 Overall System ...................................................................................................... 84 Figure 8-2 Sterilization chamber assembly............................................................................. 86 Figure 8-3 Cut-away view of sterilization chamber ............................................................... 87 Figure 8-4 Butterfly valve ....................................................................................................... 92 Figure 8-5 Reaction chamber .................................................................................................. 94 Figure 8-6 - Reaction chamber lid .......................................................................................... 95 Figure 8-7 Reaction chamber bottom ..................................................................................... 95 Figure 8-8 External case ......................................................................................................... 99 Figure 9-1 Physical Electrical Schematic ............................................................................. 101 Figure 9-2 Overall electrical diagram ................................................................................... 102 Figure 9-3 Power Supply input ............................................................................................. 103 Figure 9.9-4 Voltage Regulator ............................................................................................ 104 Figure 9-5 Sensor circuit diagram......................................................................................... 105 Figure 9-6 PX139 Pressure Sensor ....................................................................................... 105 Figure 9-7 Control Diagram 1............................................................................................... 107 Figure 9-8 Control Diagram 2............................................................................................... 108 Figure 9-9 Strip Heater ......................................................................................................... 109 Figure 9-10 Thermostat......................................................................................................... 109 Figure 9.9-11 LED ................................................................................................................ 109 Page 5 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 9-12 Wavelength TEC control WEC3243 ................................................................. 110 Figure 9-13 Thermoelectric Cooler ...................................................................................... 111 Figure 9-14 Peristaltic pump controller connection schematic............................................. 113 Figure 9-15 Controller layout ............................................................................................... 114 Figure 9-16 APEX PA75CC ................................................................................................. 114 Figure 9-17 On board computer, an embedded PC104 controller from Kontron ................. 116 Figure 9-18 Data Acquisition................................................................................................ 117 Figure 9-19 Wire Diagram .................................................................................................... 119 Figure 9-20 Switch board...................................................................................................... 119 Figure 10-1 Simplified execution ......................................................................................... 125 Figure 10-2 Autoclave Sensor Diagram ............................................................................... 127 Figure 10-3 Reaction Chamber Sensor Diagram .................................................................. 128 Figure 10-4 LabView vi Autoclave Temperature Control .................................................... 129 Figure 10-5 Software Function Tree ..................................................................................... 129 Figure 11-1 Assembly Flow Diagram................................................................................... 131 Figure 11-2 Overall Assembly .............................................................................................. 132 Figure 11-3 Autoclave Functional Test Plan ........................................................................ 133 Figure 11-4 Reaction Chamber Functional Test Plan ........................................................... 133 Figure 11-5 DAQ Functional Test Plan ................................................................................ 134 Figure 13-1 Organizational Responsibilities ........................................................................ 141 Figure 13-2 Work Breakdown Structure............................................................................... 142 Figure 13-3 Schedule for Spring Semester ........................................................................... 143 Page 6 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 List of Tables Table 1-1 Mars/Earth Comparison.......................................................................................... 14 Table 1-2 MiDAs Design Requirements................................................................................. 14 Table 2-1 Experiment Timeline .............................................................................................. 24 Table 3-1 Driving Requirements ............................................................................................ 27 Table 3-2 System Architecture Pros and Cons ....................................................................... 28 Table 3-3 Design Alternative Quantitative Analysis .............................................................. 35 Table 4-1 Driving PDD Requirements ................................................................................... 37 Table 5-1 Wetted Material Properties ..................................................................................... 42 Table 5-2 Electric Heating Options ........................................................................................ 45 Table 5-3 Insulation Options .................................................................................................. 48 Table 5-4 Temperature Sensor Comparison ........................................................................... 51 Table 5-5 Pressure Sensor Comparison .................................................................................. 52 Table 6-1 Power Summary ..................................................................................................... 69 Table 6-2 Power Summary (1st Autoclave)............................................................................ 70 Table 6-3 Power Summary (2nd Autoclave) .......................................................................... 70 Table 6-4 Component List ...................................................................................................... 73 Table 7-1 Risk Assessment Chart ........................................................................................... 81 Table 9-1 Sensor system accuracy ........................................................................................ 106 Table 9-2: TEC Pin values .................................................................................................... 111 Table 9-3: TEC specifications .............................................................................................. 112 Table 9-4 Mixer motor specifications ................................................................................... 115 Table 9-5 DAQ system ......................................................................................................... 118 Table 9-6 Drawing Tree ........................................................................................................ 120 Table 9-7 MiDAs Powered Items ......................................................................................... 121 Table 9-8 Electronics Parts List ............................................................................................ 122 Table 10-1: One cycle control............................................................................................... 126 Table 12-1 Design Requirement Verification Plan ............................................................... 136 Table 13-1 Overall Budget.................................................................................................... 145 Page 7 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 List of Acronyms A AC A/D ADP AES Al ASTID ASV BCL BST C&DH CDR CFO CFU CJC COTS CPU CP CU CUB D DAC DAQ DC DNA dH2O dO2 DO DU EC ECTFE ETFE FE FEP FFR g GCMS GEX H2O I I&T ISE JSC Analysis (verification method) Alternating Current Analog to Digital Converter Acceptance Data Package Aerospace Engineering Sciences Aluminum Astrobiology Science and Technology Instrument Development Anodic Stripping Voltammetry Bioanalytical Core Lab BioServe Space Technologies Command and Data Handling Critical Design Review Chief Financial Officer Colony Forming Unit Cold Junction Compensator Commercial Off the Shelf Central Processing Unit Chronopotentiometric University of Colorado University of Colorado at Boulder Demonstration (verification method) Digital to Analog Converter Data Acquisition Direct Current Deoxyribonucleic Acid Distilled water Dissolved Oxygen Digital Output Digital Input Electric Conductivity Ethylene-Chlorotrifluoroethylene Ethylene-Tetrafluoroethylene-copolymer Fabrication Engineer Fluoroethylene-Propylene Fall Final Report Gram, Earth Gravity Gas Chromatograph/Mass Spectrometer Gas Exchange Water Current, Inspection (verification method) Integration and Test Ion Selective Electrode Johnson Space Center Page 8 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis kg LCP LED LR m MDA MEA MECA MEL MiDAs MIL-STD mL MOSFET N/A NAI NAR NASA ORP PAB PAI PCB PCTFE PDD PDR PEEK PEI PFA pH PI PID PM P/N PPS PR PSU PTFE PV PVA PVC PVF PWM R RF RFA RFA MiDAs December 18th, 2006 kilogram Liquid Crystal Polymer Light Emitting Diode Labeled Release Mass, meter Microbial Detection Array Microelectrode Array Mars Environmental Compatibility Assessment Master Equipment List Microbial Detection Arrays Military Standard Milliliter Metal-Oxide-Semiconductor Field-Effect Transistor Not Applicable NASA Astrobiology Institute Not a requirement National Aeronautics and Space Administration Oxidation-Reduction Potential Project Advisory Board Polyamide-Imide Printed Circuit Board Polychloro-Trifluoroethylene Project Definition Document Preliminary Design Review Polyetherketone Polyetherimide (Ultem) Perfluoralkoxy Potential Hydrogen Polyimide Proportional Integral Derivative (controls) Project Manager Product Number Polyphenylene Sulfide Pyrolytic Release Polysulfone Polytetrafluoroethylene Photovoltaics Photovoltaic Array Polyvinyl Chloride Polyvinyl-Fluoride Pulse Width Modulation Resistance Radio Frequency Recommendation for Action Request for Action Page 9 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis RNA RPM RTD SE SS SW T TEC TOC TRL TRR USB V WBS WCL MiDAs December 18th, 2006 Ribonucleic Acid Revolution Per Minute Resistance Temperature Detector Systems Engineer Stainless Steel Software Test (verification method) Thermoelectric Cooler Total Organic Carbon Technology Readiness Level Test Readiness Review Universal Serial Bus Voltage Work Breakdown Structure Wet Chemistry Laboratory Page 10 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 1.0 Project Overview and Requirements Author: Shayla Stewart Page 11 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 1.1.0 Objective Microbial Detection Arrays (MiDAs) is a component of a larger project focusing on a future Mars astrobiology mission with an objective to accomplish electrochemical sensing of metabolic activity in Martian soil. The overall project, funded by an Astrobiology Instrument Development grant, consists of three research activities: biology testing through NASA Johnson Space Center (JSC), electrochemical sensing technologies of metabolic activity through Tufts University, and instrument hardware, which is the focus of this Senior Project. The MiDAs team objective is to design and build an integrated field instrument that supports the JSC and Tufts activities above, with meaningful biological and spaceflight constraints, such as autonomy, size, weight, and materials selection. The project will extend the proof-ofconcept from the laboratory to the field, in an integrated unit, and thus raise the Technology Readiness Level (TRL) from 1-3 to 4-5. The NASA TRLs are defined in Figure 1-1. This Earth-based instrument will validate the key functions set forth by the customer, BioServe Space Technologies, and will enable self-contained, integrated biology research in the lab (TRL-4) and the field (TRL5) with the electrochemical sensors from Tufts University. Figure 1-1 NASA Technology Readiness Level Definitions 1.2.0 Instrument Operation Figure 1-2 shows a simplified functional diagram of the MiDAs instrument. MiDAs operations begin with its Startup phase when the instrument begins to draw power and takes initial temperature and pressure readings through the Data Acquisition (DAQ) system. Once this is complete, a geological sample is split evenly and added into two identical sterilization chambers with a small amount of water for steam sterilization. The instrument then enters the Sterilization phase. Once the split samples are individually sterilized, they are transported into the two reaction chambers, which consist of a test and control chamber. Once the samples reach the reaction chambers, they are mixed with sterile water and the electrochemical sensors within the chamber measure an initial baseline as any soluble Page 12 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 materials reach an equilibrium. An inoculation sample, which has not been sterilized, is then added into the test chamber and the Testing phase begins under controlled thermal conditions. This phase continues for approximately 14 days. Any metabolic activity in the inoculated test chamber should be detectable by changes in the electrochemical sensor readings relative to the sterile control chamber. The growth or incubation period is envisioned to last up to 2 weeks. The experiment timeline will be discussed in more detail later in this document. The scope of this project is to design, build, and validate an integrated instrument to enable these functions and measurements, but the actual biology experimentation is outside the scope of this project. Figure 1-2 MiDAs Operational Diagram 1.3.0 Mars/Earth Comparison Table 1-1 shows a comparison of the theoretical Mars mission and the MiDAs Earth field instrument. Complete autonomy is very important for a Mars-based instrument, while this is not a primary goal for this project. The bold and italicized rows show where the Earth instrument differs from a theoretical flight-instrument. These are areas where autonomy has been lost and there is increased reliance on the experimenter to open valves and input the samples. In these cases, autonomy has been traded for reliability in the field instrument. Autonomy inherently adds expense, complexity, and failure modes without proving key Page 13 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 concepts or raising the TRL. The gray indicates the key components where autonomy is vital. It is noted that the MiDAs instrument meets the key autonomous components. The Mars instrument will, however, be able to draw on several rover/lander resources such as power, data storage, thermal control, and communications, which are all required functions for the field instrument. Given the limited resources and time for the Senior Projects design phase, there is a careful balance between proving key technology and facilitating integrated field testing. Table 1-1 Mars/Earth Comparison Theoretical Mars Mission Receive low power from Rover/Lander Receive startup command from uplink Rover/lander opens Autoclave lid Rover/lander inputs sample Rover/lander closes Autoclave lid MiDAs Earth Based Apparatus Receive low power from external source Press power button Person opens Autoclave lid Person inputs sample Person closes Autoclave lid Autoclave cycle begins through SW run Autoclave cycle begins command Reaction chamber environment controls begin Reaction chamber environment controls begin Valve opens Person opens valve Water flushes sample out of autoclave Water flushes sample out of autoclave Valve closes Person closes valve Mixing begins Mixing begins through SW run command DAQ begins DAQ begins through SW run command Inoculation sample added Person adds inoculation sample DAQ runs for 14 days DAQ runs for 14 days Data downlink from rover to satellite to Earth Data stored on-board, transfer to PC 1.4.0 Design Requirements Table 1-2 shows the MiDAs design requirements as defined in the Project Definition Document (PDD). Table 1-2 MiDAs Design Requirements Requirement # Title Requirement PDD 4.1 Reaction Chamber Volume MiDAs shall have two chambers with a minimum internal volume of 50 mL each. PDD 4.2 Reaction Chamber Temperature Each reaction chamber shall be controllable within a range of 4°C to 37°C with an accuracy of ±1°C. PDD 4.3 Reaction Chamber Pressure Each reaction chamber shall be sealed to a pressure of 1 psi differential from ambient pressure. Page 14 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 PDD 4.4 Reaction Chamber Sensor Capability PDD 4.5 Reaction Chamber Mixing Capability PDD 4.6 Reaction Chamber Multi-Use Port PDD 4.7 Reaction Chamber Material Each reaction chamber shall be manufactured out of a list of materials provided by BioServe. This list includes, but is not yet limited to, Polysulfone, PharMed, 316 stainless steel, and Ultem 1000. PDD 4.8 Geological Sample Volume Each reaction chamber shall receive no less than 5 mL and no more than 25 mL of geological sample. PDD 4.9 Inoculation Sample Volume The test chamber shall receive a maximum of 1 mL of inoculation sample. PDD 4.10 Inoculation Sample Reception The test chamber shall receive the inoculation sample through established aseptic techniques. PDD 4.11 Reaction Sample Handling The reaction samples shall be sterilized in accordance with standard Autoclave techniques. PDD 4.12 Inoculation Sample Handling The inoculation sample shall not be sterilized. The sample shall remain in the condition in which it was gathered until it is introduced into the test chamber. PDD 4.13 Reaction Sample Delivery One pre-measured reaction sample shall be delivered to the test chamber and one pre-measured reaction sample shall be delivered to the control chamber. Both samples shall maintain sterility throughout delivery. PDD 4.14 Inoculation Sample Sterility The inoculation sample shall be aseptically delivered to the test chamber. PDD 4.15 Reagent Water Containment The sterile reagent water shall be completely contained in both solid and liquid form. PDD 4.16 Reagent Water Delivery The MiDAs shall aseptically deliver no more than 50 mL (within ± 5% accuracy) of sterile reagent water to each reaction chamber. PDD 4.17 Reagent Water Temperature The reagent water shall be delivered to the reaction chambers at a temperature not to exceed 60°C. PDD 4.18 Sensor Integration The electrochemical sensors shall be placed at a minimum height within the reaction chambers to mitigate sample sedimentation effects. This height shall be sufficient to allow the sensors to be fully submerged with a minimum of 5 mL to 10 mL of fluid. PDD 4.19 Sensor Data Collection Rate The electrochemical sensors shall have a data collection rate of 1 measurement per minute per sensor. Sensor Data Acquisition Sensor Data Accessibility All data taken through the sensors shall be collected and stored for analysis. The scientific and engineering status data shall be accessible to users throughout the experiment. PDD 4.22 MiDAs Status Warnings MiDAs shall provide caution, warning, and instrument status to external ground support equipment. PDD 4.23 MiDAs Command MiDAs shall receive commands from external ground support equipment. PDD 4.20 PDD 4.21 Each reaction chamber shall be capable of supporting no fewer than 6 and no more than 18 electrochemical sensors. Each reaction chamber shall have mixing capability such that each geological sample is evenly distributed within the fluid while movement is present at each sensor location. Each reaction chamber shall have a minimum of four multi-use ports for gas and liquid exchange. Page 15 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 PDD 4.24 Field Power MiDAs shall be capable of receiving between 10 W and 30 W from an external power supply in a field setting. PDD 4.25 Laboratory Power MiDAs shall be capable of receiving between 10 W and 30 W from an external power supply in a laboratory setting. PDD 4.26 PDD 4.27 Nominal Power Consumption Peak Power Consumption Nominal power consumption shall not exceed 30 W. Peak power consumption shall not exceed 30 W for more than 30 seconds. PDD 4.28 Unit Disassembly MiDAs shall be able to be taken apart so that it may be sterilized and reassembled for multiple Earth tests. PDD 4.29 Operational Cycle One operational testing cycle shall be 14 standard Earth days, not including power-up, sterilization, and power-down. PDD 4.30 Operational Environment MiDAs shall be able to operate in environments ranging from Antarctica to Atacama Valley in Chile. 1.4.1.0 Requirement Importance 4.1 Reaction Chamber Volume The instrument must have two reaction chambers so that there can be a test and control simultaneously. This is important for the scientists using the instrument so that they can discern what changes in temperature or electrochemical activity occur naturally and which are due to potential metabolic activity in the test chamber. 4.2 Reaction Chamber Temperature Each reaction chamber must be controllable to a user-selectable set point between 4°C and 37°C and maintained constant at this set point to +/- 1°C. This is assumed to be the potential range of acceptable growth environments to metabolize and reproduce. The temperature must be controlled at the desired set point to reduce thermal effects on the sensors and ensure their long-term stability. 4.3 Reaction Chamber Pressure Each reaction chamber must be sealed to 1 psi differential from ambient in order to minimize the gas exchange that might occur between the reaction chambers and the outside environment, and as a verification method to ensure that there is no fluid leakage and to maintain sterility. 4.4 Reaction Chamber Sensor Capability Each reaction chamber has to be able to accommodate the appropriate number of electrochemical sensors provided by Tufts University in order to collect the desired amount of measurements. 4.5 Reaction Chamber Mixing Capability The fluid in the reaction chambers must be mixed as uniformly as possible so that the sensor readings are accurate. If there is a lot of sample sedimentation, then the solution will not be homogeneous and the sensors may not be detecting all of the potential metabolic Page 16 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 activity. There must also be fluid movement at each of the sensors in order to break down the boundary layer, which is necessary for the sensors to take accurate readings. 4.6 Reaction Chamber Multi-Use Port Having at least four multi-use ports makes it possible for the user to input/output gas or liquid to/from the reaction chambers during the experiment. 4.7 Reaction Chamber Material The list of possible reaction chamber materials is comprised of materials that can all withstand autoclaving, so that they can be sterilized, have high resistance to corrosion, since the sample/water solution may be corrosive, are approved for food and pharmaceutical applications, and are inert so that there is no chemical reaction between the chamber and the solution within it. 4.8 Geological Sample Volume The geological sample must be at least 5 mL, which provides enough material to obtain measurable results. This is a customer specification. The maximum volume of 25 mL is imposed so that a range of different sample sizes can be accommodated during field testing and concept evaluation. 4.9 Inoculation Sample Volume The inoculation sample volume should be less than or equal to 1 mL, which is much smaller than the reaction sample, so that any reactions that may take place in the test chamber are not creating false positive readings. The chemistry of the reaction samples may be altered by autoclaving, and a large volume of non-sterilized yet inert inoculation sample could create electrochemical changes. 4.10 Inoculation Sample Reception The inoculation sample must be aseptically delivered to the reaction chambers so that the user knows that any detected metabolic activity is from life forms already present in the sample, not transferred to the sample during transportation. 4.11 Reaction Sample Handling The reaction samples will be sterilized through steam autoclaving, with options for multiple autoclave cycles upon customer request. This is the sterilization method with the best chance of killing most known forms of life (e.g. bacteria, viruses, spores, etc.). 4.12 Inoculation Sample Handling The inoculation sample must remain unsterilized so that any potential living organism present is preserved. 4.13 Reaction Sample Delivery Having equal amounts of sample in each reaction chamber helps maintain uniformity between the test and control. Once the sample is sterilized, it has to remain sterile so that no life forms are introduced. Page 17 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.14 Inoculation Sample Sterility The inoculation sample must not become contaminated with any living organisms from the MiDAs instrument. If metabolic activity is detected, the experimenter needs to know that the living organisms were originally in the sample or the experiment becomes obsolete. 4.15 Reagent Water Containment The reagent water must be contained in both solid/frozen and liquid form so that the instrument can be transported at a range of temperatures without being powered. Prior to use, if the instrument is operated in a cold ambient environment, the water must be heated to be a liquid. 4.16 Reagent Water Delivery The reagent water delivery must be contained and transported aseptically, so that no living organisms are transferred to the reagent water. 4.17 Reagent Water Temperature The electrochemical sensors provided by Tufts University cannot withstand temperatures above 60°C, so the water must be delivered below this temperature. For delivery, it must be liquid and thawing may be required prior to use. 4.18 Sensor Integration The sensors must be able to take readings when the minimum amount of sample/water solution is in the chamber, so the sensors must be completely submerged in 510 mL. 4.19 Sensor Data Collection Rate Since the experiment takes place over 14 days, a reading each minute from each sensor is sufficient to characterize the experiment results. 4.20 Sensor Data Acquisition The data must be collected and stored so that the experimenter can obtain and study the data after the experiment is completed. 4.21 Sensor Data Accessibility The experimenter should be able to access the data during the experiment so as to observe the status of the experiment while it is in progress. 4.22 MiDAs Status Warnings Caution, warning, and status signals are necessary to be able to observe the instrument’s status as well as detect errors throughout the experiment. 4.23 MiDAs Command The duration of the experiment is such that it is not reasonable to have the user initiate each step of the process, so the instrument must be capable of receiving software commands. Page 18 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.24 Field Power This 30 W power requirement helps pave the way for future space missions. This is based on predicted wattage for future Mars rover missions. 4.25 Laboratory Power The instrument needs to be capable of running in a laboratory, as well as the field. 4.26 Nominal Power Consumption The 30-Watt nominal power consumption is based on predicted wattage provided by future Mars rovers. 4.27 Peak Power Consumption The Mars rovers can provide more than nominal power for brief, scheduled periods of time, so the peak power consumption must not exceed 30 W for more than 30 seconds. 4.28 Unit Disassembly The instrument must be reusable, so disassembly must be possible for sterilization purposes. 4.29 Operational Cycle The 14-day operational testing cycle is the time allotted for the potential living organisms to reproduce and metabolize. 4.30 Operational Environment Antarctica and Atacama Valley, Chile are the potential test sites for the MiDAs instrument, so it must be designed for these environmental conditions. These conditions include an ambient temperature range between -10°C and 40°C, ambient pressures as low as 70,000 Pa (3000 meter altitude) and a relative humidity of 0-95%, which is just below the condensation percentage. 1.5.0 Deliverables The deliverables from the MiDAs senior project team to the customer is the field-ready unit with a TRL of 4-5 with test data verifying the requirements listed in Table 1-2. The customer will also receive an operational manual outlining how to assemble/disassemble the instrument as well as how to operate various components. There will also be a document proposing design solutions to further raise the TRL to 6-7. This design document will include ways to increase autonomy in the areas outlined in bold italics in Table 1-1. Page 19 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 2.0 System Architecture Author: Sameera Wijesinghe Page 20 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Upon analyzing the customer requirements as described in detail in the previous section, the following design was developed. Figure 2-1 below shows the overall system architecture for MiDAs. Since sterility was a main concern for this project, the MiDAs unit was designed to be completely disassembled in a short period of time. This gives the experimenter the added flexibility of being able to sterilize all the experimental related components in an external autoclave prior to carrying out any experiments. The relative ease of the disassembly process can further be seen in the figure below. Embedded CPU DAQ Figure 2-1 Overall System Architecture It is important to note that the insulation as well as few other non critical components has been removed for clarity. Highlighted in Figure 2-2 are all the major components that make up the MiDAs unit. MiDAs receives 12V from an external power source which will provide power to the motors, pumps and the heaters. Using a voltage regulator the 12V will be reduced to 5V to accommodate the electrochemical sensors which are provided by the customer. Since the temperature in both the autoclaves and also the environment chamber (see figure 2-2 below) needs to be actively controlled to 1˚, an embedded CPU will be utilized which operates on custom software for this purpose. Page 21 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis Peristaltic Pump MiDAs December 18th, 2006 Water Chamber Heat Sink Autoclave Valve PharMed Tubing Environment Chamber Test Chamber Cap Mixer Sensor Ports Test Chamber Base Motor Shaft Motor Base Figure 2-2 Internal Components 2.1.0 Critical Components 2.1.1.0 Autoclaves Each autoclave consists of three strip heaters and a thermoelectric cooler (TEC). The TEC will only be used in cooling the autoclaves instead of aiding in the process of heating, although this configuration could be easily changed during adverse weather conditions. Furthermore, each autoclave will consist of a temperature sensor and a pressure sensor located inside. 2.1.2.0 Peristaltic Pumps There are two peristaltic pumps (one per autoclave), each capable of precisely metering out 25mL of reagent water. Page 22 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 2.1.3.0 MiDAs December 18th, 2006 Reagent Water The peristaltic pumps described above will be used in drawing precisely the required volume of liquid from the water chamber and pumping it directly into the autoclave chambers once the autoclaves has completed the sterilization process. 2.1.4.0 Valve The butterfly valve which is affixed to the base of the autoclave will open manually through user input once the sterilization has been completed in the autoclaves. 2.1.5.0 Test Chambers Once the sterilized soil sample has been received by the two chambers the sensors located along the perimeter of the chambers will acquire data once every minute to detect any metabolic activity in the sample. 2.1.6.0 Mixers The custom designed mixers will ensure that no sedimentation of the sample occurs at the base of the test chambers. The mixers will be continuously operated so that the data acquired by the sensors will not be subjected to any ambiguity. 2.1.7.0 Tubing PharMed tubing will transport the mixture of water and soil sample into the test chambers. The internal diameter of the tubing is approximately one inch and extends directly downwards for up to three inches to ensure that the soil transportation occurs with minimal amount of interference. 2.1.8.0 Motors Two low power motors will be utilized to spin the custom designed mixers. The motors will operate continuously for the entire duration of the experiment once the sample has been transported into the test chambers Page 23 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 2.2.0 Experiment Timeline The sample will be inserted into the two autoclaves by the experimenter and then sealed. The melamine insulation will be placed over the two autoclaves and the environment chamber to prevent heat loss. The required amount of insulation of two inches was based on the thermal conductivity of melamine. There will be total heat loss of 1.6W though the insulation. Once the insulation is in place the software will be enabled by the experimenter to begin the experiment. MiDAs unit is required to operate under various ambient conditions. Due to this reason it should be noted that the times shown below in Table 2-1 are for the worst case scenario. Table 2-1 Experiment Timeline Subsystem Description Autoclave Raise the temperature of the sample from ambient to 121˚C Hold at or above 121˚C Cool the sample to between 4°C and 37˚C Hold between 4°C and 37°C Repeat three more cycles (Step1-4) 3.851 0.25 3.232 24 68.32 Accept the soil sample from the autoclaves and begin the testing process 336 Reaction Chambers Time (hours) 2.3.0 Electrical Subsystem Figure 2-3 shows the various electrical interconnections with the hardware components of MiDAs. There are a total of 10 sensors that will be provided by MiDAs for measuring temperature and pressure. The electrochemical sensor package which consists of 24 sensors will be provided by Tufts University. 1 2 Time based on an ambient temperature of -10°C Time based on a cooling temperature of 20°C Page 24 of 196 Computer x 2 Distribution MiDAs December 18th, 2006 Power Supply Power Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis x 3 6 Input Analog x 0 1 x 0 output x 1 1 x Output x x 4 x 2 1 TEC x 1 TEC AC 2 TEC 2 TEC AC 1 TEC RC Autoclave Autolcave x 4 2 x 4 x 1 Control 2 Control 2 TEC RC RC RC 2 H2 For optional proportional control Autoclave x 2 x 4 x 2 LED's x 2 H1 x 3 1 Control 1 Mixer 2 Mixer 2 Control Mixer Mixer x 2 Autoclave x 2 x 3 x 2 Pump1 x 2 Pump 2 6 Analog x 2 Digital Board Switch Number of wires 2 2 Sensors DAQ Figure 2-3 Electrical Subsystem In addition to the sensors the two peristaltic pumps, the two motors, and the heaters/coolers, need to be connected to the switch board for on/off control. If proportional control of the motors or TEC is requested by the customer the mixer motors and TEC can be connected to the analog outputs of the DAQ. Page 25 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 3.0 Development and Assessment of System Design Alternatives Author: Shayla Stewart Page 26 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 When developing system design alternatives, there were several driving requirements considered. These requirements are listed in Table 3-1. The design alternatives examine the number of sterilization and reaction chambers necessary to fulfill these requirements. This is a fundamental issue for the MiDAs team because the number and layout of the chambers drive the thermal analysis, power budget, mass, volume, and cost of the overall instrument. There were five overall system architectures considered for this instrument. Table 3-1 Driving Requirements Requirement # Requirement PDD 4.1 Reaction Chamber Volume PDD 4.2 Reaction Chamber Temperature PDD 4.3 Reaction Chamber Pressure PDD 4.5 Reaction Chamber Mixing Capability PDD 4.7 Reaction Chamber Material PDD 4.8 Geological Sample Volume PDD 4.11 Reaction Sample Handling PDD 4.13 Reaction Sample Delivery PDD 4.24 Field Power PDD 4.25 Laboratory Power PDD 4.26 Nominal Power Consumption PDD 4.27 Peak Power Consumption PDD 4.29 Operational Cycle There were two main parameters considered when establishing the five system architecture options. The architecture of the sterilization chambers was the first considered and there were three main options examined. The sample could be sterilized within the reaction chamber, there could be one separate chamber for sterilization of the geological sample, or there could be two separate sterilization chambers for each the test and control samples. The second parameter considered was the environmental controls for the two reaction chambers. Both the test and control chambers must be maintained at a given pressure and temperature throughout the testing phase as stated in PDD requirements 4.2 and 4.3. Both reaction chambers could have separate environmental controls or they could share the same environment. Separate environments would involve sealing each chamber separately and having thermal control and insulation around each chamber. In a shared environment, the test and control chambers would be placed together inside a larger environmental chamber. This larger chamber would be thermally controlled and insulated, but the reaction chambers themselves would not. 3.1.0 System Architecture Pros and Cons The geological sample has to be sterilized using steam autoclave techniques. Using a single autoclave for the entire sample would involve splitting the sample into two equal volumes to be transferred into the two reaction chambers once the sterilization is complete. If two separate autoclaves were used, the sample would be split into equal volumes and placed in Page 27 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 the autoclaves before sterilization begins. Another decision is whether or not to have a shared environmental control for both reaction chambers or whether to have one for each chamber. With some of the overall system architecture options outlined later on, several environmental sensors (i.e. temperature and pressure sensors) would be necessary in order to obtain accurate measurements. There are others that would involve the use of only a few environmental sensors. In either case, the sensors would have to be located in optimum such a way as to minimize interference with other components within the MiDAs instrument. The electrochemical sensor package provided by Tufts University has a maximum temperature limitation of 60°C. As mentioned previously, standard autoclave techniques require a temperature of at least 121°C. This means that the electrochemical sensors cannot be within the autoclave during operation. Therefore, if the sample were to be sterilized within the reaction chamber, some sort of moving sensor package would have to be developed. This way, the sensors could be moved into the chamber once the sterilization is completed. If the sterilization and reaction chambers are separate entities, a fixed sensor package in the reaction chambers would be used. Table 3-2 shows the pros and cons for each of the system architecture decisions including the number of autoclaves, number of sensors, whether to have a moving or fixed sensor package, and whether or not to have a shared environment for the reaction chambers. Table 3-2 System Architecture Pros and Cons Option Separate Autoclave Shared Autoclave Few Environmental Sensors Several Environmental Sensors Moving Sensor Package Fixed Sensor Package Separate Environment Shared Environment Pros Eliminates need to split sample into equal parts Fewer parts Less machining time Fewer parts Less power consumption Sensor redundancy No sample transport necessary - no contamination Reduced complexity – no extra moving parts Smaller operational environment – easier to thermally control Easy to keep test and control under same conditions Cons More machining time Need to split sample into equal parts No redundancy – failure compromises experiment Additional parts More power consumption Very complex Failure renders experiment useless Sample transport required – chance for contamination Difficult to keep test and control under same conditions Additional parts Larger environment – more power to thermally control Page 28 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 3.2.0 System Architecture Options 3.2.1.0 Option A – Monobox Description This system architecture utilizes a single chamber for sterilization and the experimental testing. There are two reaction chambers within the autoclave for the test (marked “Exp” for experiment) and control. The testing is performed in both chambers at the same time. Figure 3-1 shows a functional schematic of this system architecture. This option has the pros and cons of a shared autoclave, few sensors, a moving sensor package, and a shared environment as defined in Table 3-2. Figure 3-1 Option A Schematic Experimental Timeline Stage 1: Receive inoculation sample and geological sample from outside influence Stage 2: Add the geological sample to each or the reaction chambers (equal parts) Stage 3: Autoclave to sterilize both reaction chambers Stage 4: Cool and hold for 24 hrs Repeat Stages 3 and 4 twice more Stage 5: Add reagent water to each reaction chamber (equal parts water for both chambers) Page 29 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Stage 6: Use the mixer to fully mix both chambers (the mixer will be used throughout the duration of the experiment) Stage 7: Homogenize the environment of the chambers using the environmental controls Stage 8: Add the inoculation sample to the test chamber Stage 9: Add the sensor package to each of the reaction chambers Stage 10: Test for two weeks 3.2.2.0 Option B – Single Sterilization, Shared Environment Description The geological sample in this option is sterilized in a single chamber and divided equally into the test and control chambers. The test and control chambers are both inside an enclosed space with shared environmental controls. Figure 3-2 shows a functional schematic of this system architecture. This option has the pros and cons of a shared autoclave, few sensors, a fixed sensor package, and a shared environment as defined in Table 3-2. Figure 3-2 Option B Schematic Experimental Timeline Stage 1: Receive inoculation sample and geological sample from outside influence Stage 2: Add the geological sample to the autoclave Page 30 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Stage 3: Autoclave to sterilize sample Stage 4: Cool and hold for 24 hrs Repeat Stages 3 and 4 twice more Stage 5: Separate the sterilized sample into equal parts Stage 6: Add each sterilized sample to the reaction chambers Stage 7: Add equal parts reagent water to the reaction chambers Stage 8: Homogenize the environment of the chambers using the environmental controls Stage 9: Add inoculation sample to the test chamber Stage 10: Test for two weeks 3.2.3.0 Option C – Single Sterilization, Separate Environment Description Like Option B, the samples for both reaction chambers are sterilized in a single sterilization chamber, however, after sterilization, the sample is divided into two separate test and control chambers with separate environmental controls. Figure 3-3 shows a functional schematic of the system architecture. This option has the pros and cons of a shared autoclave, several sensors, a fixed sensor package, and a separate environment as defined in Table 3-2. Figure 3-3 Option C Schematic Experimental Timeline Stage 1: Receive inoculation sample and geological sample from outside influence Page 31 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Stage 2: Add the geological sample to the autoclave Stage 3: Autoclave to sterilize sample Stage 4: Cool and hold for 24 hrs Repeat Stages 3 and 4 twice more Stage 5: Separate the sterilized sample into equal parts Stage 6: Add each sample to the reaction chambers Stage 7: Add equal parts reagent water to the reaction chambers Stage 8: Homogenize the environment of the chambers using the environmental controls Stage 9: Add inoculation sample to the test chamber Stage 10: Test for two weeks 3.2.4.0 Option D – Dual Sterilization, Separate Environment Description This option utilizes two separate sterilization chambers. After sterilization, the sample from each sterilization chamber is placed in a reaction chamber, and each reaction chamber has its own environmental controls. Figure 3-4 shows a functional schematic of the system architecture. This option has the pros and cons of a separate autoclave, several sensors, a fixed sensor package, and a separate environment as defined in Table 3-2. Page 32 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 3-4 Option D Schematic Experimental Timeline Stage 1: Receive inoculation sample and geological sample from outside influence Stage 2: Add the geological samples to each autoclave Stage 3: Autoclave to sterilize samples Stage 4: Cool and hold for 24 hrs Repeat Stages 3 and 4 twice more Stage 5: Add the sterilized samples to the reaction chambers Stage 6: Add equal parts reagent water to the reaction chambers Stage 7: Homogenize the environment of the chambers using the environmental controls Stage 8: Add inoculation sample to the test chamber Stage 9: Test for two weeks 3.2.5.0 Option E – Dual Sterilization, Shared Environment Description This option also has two separate sterilization chambers. The sample from each sterilization chamber is placed into separate reaction chambers, but the chambers are kept together in a shared environment. Figure 3-5 shows a functional schematic of the system architecture. This option has the pros and cons of a separate autoclave, few sensors, a fixed sensor package, and a shared environment as defined in Table 3-2. Page 33 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 3-5 Option E Schematic Experimental Timeline Stage 1: Receive inoculation sample and geological sample from outside influence Stage 2: Add the geological samples to each autoclave Stage 3: Autoclave to sterilize samples Stage 4: Cool and hold for 24 hrs Repeat Stages 3 and 4 twice more Stage 5: Add the sterilized samples to the reaction chambers Stage 6: Add equal parts reagent water to the reaction chambers Stage 7: Homogenize the environment of the chambers using the environmental controls Stage 8: Add inoculation sample to the test chamber Stage 9: Test for two weeks 3.3.0 Quantitative Analysis Option A, which involves having the sterilization and testing phases in the same chamber, was eliminated early on in the design phase due to the fact that it adds complexity with the necessity of a moving sensor package. The mass, volume, and cost of each of the Page 34 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 four remaining options were considered as shown in Table 3-3, although the estimates were far too rough to use in the final system architecture decision. The driving requirements listed in Table 3-1 became far more important than these three quantities as all four remaining system architecture options are reasonable within self-imposed constraints. Table 3-3 Design Alternative Quantitative Analysis Shared Environment, Shared Sterilization Separate Environment, Shared Sterilization Separate Environment, Separate Sterilization Shared Environment, Separate Sterilization Mass (g) 900 1200 1400 1300 Volume (mL) 4444 4504 4612 4592 Cost $150 $1,350 $1,750 $1,350 3.4.0 Selected System Architecture After developing and analyzing the five different system architecture options, Option E – Dual Sterilization, Shared Environment has been chosen. The decision process used is outlined in Figure 3-6. Figure 3-6 System Architecture Decision Process First, the differences between shared and separate environments were considered. Having separate environments had the advantage of more precise thermal control. The shared environment, however, had more benefits. These benefits included reduced complexity due to having only one environment to control, a broader range of possible heating options, and better possible placement of insulation and mixers. After considering the environmental options, the advantages of each sterilization chamber option were considered. Sterilizing within the test and control chambers was hard to justify because of the added complexity of moving the electrochemical sensors into the chamber after sterilization. Using only one large sterilization chamber was appealing because it only required one thermal control system, however, it also added complexity by needing to weigh and divide the sample into equal volumes after sterilization. Having two sterilization chambers eliminates the need to divide the sample, which greatly reduces the complexity of the design and fabrication. The decision to implement a shared environment and two sterilization chambers led to the decision to use Option E defined described previously. Page 35 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.0 System Design-To Specifications Author: Elizabeth Newton Page 36 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 After developing the overall system architecture, several system-level design-to requirements can be determined. These design-to requirements are driven by six PDD requirements, listed below in Table 4-1. Table 4-1 Driving PDD Requirements Requirement # PDD 4.11 PDD 4.13 PDD 4.26 PDD 4.27 PDD 4.28 PDD 4.30 Requirement Reaction Sample Handling Reaction Sample Delivery Nominal Power Consumption Peak Power Consumption Unit Disassembly Operational Environment 4.1.0 Reaction Sample Handling This requirement states that steam sterilization is the necessary sterilization procedure for the geological sample. Steam sterilization requires that the sample be heated to at least 121°C while maintaining pressure. This is an important driving requirement for the overall system. Since the ISEs cannot withstand temperatures above 60°C, using autoclave techniques meant that the sterilization and testing had to occur in separate chambers, as detailed in Chapter 3: Development and Assessment of System Design Alternatives. Several design-to requirements flow from this PDD requirement. 4.1.1.0 Environmental Chamber Because of the very high temperatures achieved by the autoclave chambers, insulation must be placed between the chambers and the ISEs. This requirement contributed to the environmental chamber design, which shields the ISEs from the heat of the autoclaves as well as heats and cools the reaction chambers. Extra insulation must also be added on top of the environmental chamber to further protect the ISEs. 4.1.2.0 Support Structure Due to the high heat and pressure of the autoclaves, they had to be made out of an approved metal, SS 316 stainless steel. They also had to have a minimum wall thickness, as detailed in Chapter 8: Mechanical Design Elements. These requirements resulted in an autoclave design that is very heavy. Also, the valve interface from the autoclaves to the PharMed tubing had to follow the PDD material requirements, and is also made from SS 316 stainless steel. It also had to withstand the high pressures of the autoclave, so the silicone seat is very sturdy. This results in a heavy valve that is fairly difficult to open and close. These requirements flowed down into the need for a sturdy support structure. In order to open and close the valves without tipping the valve and autoclave assembly, the assembly will be screwed down onto an aluminum plate, which is attached by thick aluminum spars to the bottom plate. This design is detailed further in Chapter 8. Page 37 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.2.0 Reaction Sample Delivery This requirement states that the sterilized sample must remain sterile throughout its delivery to the reaction chambers. This requirement drove the clean room assembly design-to requirement. 4.2.1.0 Clean Room Assembly In order to ensure that the sample pathway through the valve and tubing is sterile, any wetted surfaces must be autoclaved in a laboratory prior to assembly. These surfaces include the SS316 valve, PharMed tubing, Ultem 1000 reaction chamber cap, body, and floor, the impeller for the mixer, and the ISEs. The exceptions to the autoclave requirement are the ISEs, which cannot withstand autoclaving. Another sterilization method must be used for these sensors. After these items are sterilized, they must be assembled in a clean room environment. This assures that no biological material is introduced into the sterile pathway. Once these items are assembled, they form a sealed unit, which can then be taken into the field. This sealed, sterile unit is inserted into the rest of the instrument, which does not need to be assembled in a clean room. The follow-on design-to requirement from this PDD requirement is that it must be relatively simple to insert the sterile unit into the instrument without taking it apart. This necessitated the spacing of the assemblies within the chassis and the slots in the shelves, as seen in the drawings in Chapter 8. 4.3.0 Nominal and Peak Power Consumption The very low power allotment for the instrument drove many of the subsystem decisions. It also determined one system-level requirement, which is the addition of insulation throughout the chassis. 4.3.1.0 System Insulation Without the benefit of high-powered heating and cooling, every bit of thermal adjustment must be conserved. This necessitated the addition of insulation throughout the system. Both autoclaves will be heavily insulated under 2” of melamine insulation. They rest on top of an aluminum shelf, and cork insulation will be placed between the valve and the shelf to minimize thermal losses into the shelf. The environmental chamber will also be insulated with melamine insulation to limit heat loss while the reaction chamber thermal control is active. These imposed requirements will maximize the thermal control available from 30W of power. 4.4.0 Unit Disassembly Disassembly of the unit is important for sterilization. Every wetted surface that is capable of being autoclaved must be able to be removed from the unit. The design-to requirement for this PDD requirement is to make the entire unit completely able to be taken apart. This ensures that it is simple to assemble the unit before an experiment and break it down for cleaning after an experiment. It also allows access to parts for troubleshooting purposes. Page 38 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.5.0 Operational Environment The operational environment PDD requirement is two-fold; first, it implies that the instrument must be capable of performing in a field setting, and second, it defines that setting. This flows down into several requirements. 4.5.1.0 Instrument Mass and Volume Because the unit must be useable in a field environment, it must be portable. Portable is defined as being relatively easy for one person to carry. It can also be defined as the maximum size allowable as carry-on luggage on a commercial airplane. This flows into the requirement that the unit must weigh less than 40 lbs. It must also be equipped with handles to make it easier to move. These are not included in the current design, but will be added to the chassis along structurally sound points. Also, the volume of the instrument must be small enough to be carried by one person with a minimal amount of difficulty. 4.5.2.0 Instrument Cost Since this instrument designed for field use, it must be relatively inexpensive to replace parts. Damage and wear are inevitable, so minimizing the cost of components is an important design-to requirement. 4.5.3.0 Component Replacement As mentioned with instrument cost, damage and wear are inevitable. Therefore, the instrument must be designed so that parts can be replaced relatively simply. The chambers, which are a fabricated component, are quite complicated and difficult to machine. The solution to this is to make the components that are most likely to need replacing easier to machine. The caps and bottoms to both the autoclave and reaction chambers are manufactured separately and are generally simpler parts than the chamber bodies. Bearing wear on the motor can be remedied by purchasing a new collar bearing and manufacturing a new reaction chamber bottom, which is a fairly simple plastic piece. The autoclave valves are able to be disassembled so the silicone seals can be replaced if they become worn or damaged. 4.5.4.0 Instrument Reliability Field instruments must be very reliable. The inclusion of moving parts must be limited and where possible, manual backups should be available. 4.5.5.0 Field Environment As a field instrument, the unit must work in the specified environments. This caused many design-to requirements related to temperature. Every component of the instrument must be rated to function at temperatures between -10°C and 40°C. Page 39 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 5.0 Development and Assessment of Subsystem Design Alternatives Author: Ted Schumacher Page 40 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 5.1.0 Reaction Chamber 5.1.1.0 Subsystem Design-to Requirements Requirement 4.1. MiDAs shall have two chambers with minimum internal volume of 50mL. Requirement 4.2. Each reaction chamber shall be controllable within a range of 40C to 370C with an accuracy of ±1°C. Requirement 4.3. Each reaction chamber shall be sealed to a pressure of 1 psi differential from ambient pressure. Requirement 4.4. Each reaction chamber shall be capable of supporting no fewer than 6 and no more than 18 electrochemical sensors. Requirement 4.5. Each reaction chamber shall have mixing capability such that each geological sample is evenly distributed within the fluid while movement is present at each sensor location. Requirement 4.6. Each reaction chamber shall have a minimum of four multi-use ports for gas and liquid exchange. Requirement 4.7. Each reaction chamber shall be manufactured out of a list of materials provided by BioServe. This list includes, but is not yet limited to, Polysulfone, PharMed, 316 stainless steel, and Ultem 1000. Requirement 4.8. Each reaction chamber shall receive no less than 5 mL and no more than 25 mL of geological sample. Requirement 4.9. The test chamber shall receive a maximum of 1 mL of inoculation sample. Requirement 4.10. The test chamber shall receive the inoculation sample through established aseptic techniques. Requirement 4.18. The electrochemical sensors shall be placed at a minimum height within the reaction chambers to mitigate sample sedimentation effects. This height shall be sufficient to allow the sensors to be fully submerged with a minimum of 5 mL to 10 mL of fluid. Requirement 4.24. MiDAs shall provide its own power (between 10 W and 30 W) in a field setting. Requirement 4.29. One operational testing cycle shall be 14 standard Earth days, not including power-up, sterilization, and power-down. Requirement 4.30. MiDAs shall be able to operate in environments ranging from Antarctica to Atacama Valley in Chile. 5.1.2.0 Requirement Analysis The first stage in the design process for the reaction chambers was to look at requirement 4.1. which determines the first bound on the reaction chamber volume. This set a maximum volume for the reaction chamber of 50mL. Next requirement 4.2. was looked at stress the need to minimize volume to maintain an accuracy of ±1°C with our heaters. The pressure requirement 4.3 shows that they chambers need to be sealed. The reaction chambers need to be capable of supporting a maximum of 18 electrochemical sensors specific to requirement Page 41 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.4. They also need a mixing apparatus to stir the solution based on requirement 4.5. The design needs to accommodate 4 multi-use ports for gas and liquid exchange from requirement 4.6. The thickness of the reaction chamber walls is dependent upon the list of materials defined in requirement 4.7. The minimum volume bound needs to accommodate the geological sample from requirement 4.8. The minimum volume bound is increased based on requirement 4.9.2. A mode of transportation for the inoculation sample must be developed from requirement 4.10. The placement of the electrochemical sensors is specifically outlined in requirement 4.18. The reaction chambers must be able to withstand the conditions for the duration of the experiment based on requirement 4.29. Mass is based upon the need to operate the field test instrument in the remote areas stated in requirement 4.30. 5.1.3.0 Internal Volume Now that the requirements were taken into account a specific internal volume of 3.53in3 (57.92cm3) was determined from the placement of the electrochemical sensors and ensuring all the above requirements were met. The dimensional requirements are satisfied and a trade study to determine the material to use for the reaction chambers was undertaken. 5.1.4.0 Material Requirements The greatest limiting requirement for our material selection was requirement 4.7. This is a pre-selection of materials that BioServe knows to be biocompatible, inert and autoclavable. MiDAs needs to be transported to remote areas based on requirement 4.30. There is no specific mass requirement stated, but based on an assumption from Dr. Hoehn we cannot exceed a mass of 50lbs (22.68kg) to accommodate the checked baggage maximum weight for air travel. This was important in the selection and the different densities and thermal properties of our materials are shown below in table 1. Density 3 Ultem 1000 SS 316 PharMed Polysulfone 5.1.4.1.0 kg/m 1270 7990 970 1220 Table 5-1 Wetted Material Properties Autoclavable Min. Temp. Max. Temp. yes/no 0 C 0 Thermal Cond. C W/m-K yes n/a 170 0.22 yes n/a 1371 16.2 (@ 1000C) yes -51 135 n/a yes n/a 140 0.12 - 0.26 Ultem 1000 Ultem 1000 is available as rods (0.375" to 6.000” diameter) and plates (0.250" to 4.000" thick), sheets, cubes, bars, and discs at a cost of $42.98 for a 2” diameter rod and a 6” length. Ultem 1000 is used for internal components on aircraft, automobiles, pipes, valves, circuit boards, and microwave applications. It is FDA compliant, NSF certified, and USDA Page 42 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 approved. Its advantages are that it has a very high specific strength, is easy to machine, and is available in many different shapes and sizes. It also has a higher operating temperature than Polysulfone, which may allow it to be used as an autoclave chamber candidate. Ultem 1000 film also has a high impact strength and good weather resistance, which may allow it to be used as a candidate for the chassis material. It also has flight heritage from various spaceflight experiments. A disadvantage of Ultem 1000 is relatively expensive and it may not be able to be used as an autoclave chamber if the heating element is too hot. 5.1.4.2.0 316 Stainless Steel 316 SS is available as tubes, bars, sheets, pipes, many diameters and thicknesses at a cost of $100 for a 2” diameter rod. PharMed is available as tubes of at least 1 foot, with internal diameters of 1/16” to 3/4” at a cost of $12.39 for 1 foot with an internal diameter of ¾”. 316 Stainless Steel is used in food and pharmaceutical processing equipment, corrosive chemical processing, and surgical implants. 316 SS is particularly corrosion resistant due to its higher molybdenum content. It is meets various ASTM certifications. The advantages of using 316 SS are high strength, a high melting temperature, and excellent corrosion resistance. It is also readily available in many shapes and sizes. The disadvantages of using 316 SS are high density and the fact it is more expensive and more difficult to machine than plastics. 5.1.4.3.0 Polysulfone Polysulfone is available as hexagonal rods, sheets, circular rods (diameters from 0.188” – 6”) at a cost of $43.39 per foot for a 2” diameter rod. Common applications for this material include electronic equipment, semiconductor processing, food service, medical, and pharmaceutical industries (mcmaster.com). This material is FDA compliant, meets UL 94V0 standards for flammability, and is NSF certified. The advantages of using Polysulfone are its low density, high specific strength, and easy to machineability with no special equipment required. The disadvantages are that it may not be able to be placed in contact with a heating element. It is also unavailable in a wide range of shapes and sizes. 5.1.4.4.0 PharMed PharMed is flexible tubing that is resistant to chemicals and can be autoclaved. It is FDA compliant and NSF certified and used in the food and biomedical fields. Some diameters are vacuum-rated. The advantages of PharMed are its low density and it can be autoclaved. It is inexpensive and available from at least one supplier in small amounts (some suppliers require a minimum 25’ purchase). It is unable to be machined into a reaction chamber since it is purchased in tubular form. Therefore Ultem 1000 will be used to machine the test chambers. Page 43 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.4.5.0 MiDAs December 18th, 2006 Material Selection The system needs to be heated based on requirement 4.2. Due to Ultem 1000’s high thermal properties we were able to determine that we could attach heaters directly to the shared environment. Based on table 5-1, showing Ultem 1000’s thermal properties, it can withstand the temperatures that will result in the shared environment. We determined to use a shared environment based on other specifications stated in Chapter 3. 5.1.5.0 Heating Element Trade Study A trade study for determination of a heating element was conducted. Based on requirement 4.2 and control of the temperature in the reaction chambers within the given range is important. The temperature control of ±1°C will be delivered through the use of a control system, not the heating element. The geometry of the chambers and the shared environmental control area will determine how much actual surface area is available for heating The options for heating elements are listed below: 1. Heaters using electrical resistance to produce heat. 2. Heaters using a chemical change to produce heat in an exothermal reaction 3. Heat from direct solar exposure. 5.1.5.1.0 Electrical Resistance Heaters Electrical resistance heaters use the internal resistance of the heater to determine how much power is dissipated for a given voltage. A heater with a resistance of 4.8 will ideally dissipate 30W of heat when driven by a 12V supply. Electric heaters can be broken up into groups depending on the primary type of material they are used to heat. Solid surfaces, gases or liquids. The different types and information regarding them can be seen in Table 5-2. It should be noted that the heaters listed in Table 5-2 were chosen from those available off the shelf using size then power as the primary considerations. Custom heaters are available for all types that could provide an internal resistance of 4.8, the only exception to this is the Minco flexible heater. The watt density listed in the table for each heater is the wattage concentration on the heater surface. This is important for heater life considerations and material properties of the surface the heater is applied to. Higher watt densities produce higher surface temperatures and shorter heater life spans. Changes in the internal structure of the heater can change watt density while still maintaining the same total wattage output. The advantages of this option are their variety of types and configurations. Their disadvantages are that they require an electrical power source, they are very small heaters, and some types are difficult to procure. Page 44 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 5-2 Electric Heating Options Tubular Tape or flexible Immersion Gases Solid surfaces or Liquids possibly gases Cartridge Solids Omega TRI1212/120 Minco HK5464R4.9L12A Omega RI100/120 Omega CSS01235/120 29.39W at 12V 2W at 12V 0.7W at 12V 4W at 12V 14 3.3W at 12V 30 4 31 50 34 57.6 43.6 4.9 72 205.7 36 0.208 0.275 2.45 0.167 0.058 0.334 5.5 x 1 x 1.5 0.246 O.D.x 12 long 3x3 Internal heating component = tube 1.5 long x 0.625 O.D. 0.124 O.D. x 2 long 1.25 I.D. x 1.5 width Weight of example (lbs) Price of example Advantages 0.4 0.2 0.01 3 0.06 0.87 $30 $28 $33.80 $115 $26 $32 Strong sheath Good at heating air Strong sheath Difficult to find small sizes Custom length and resistance needed Direct heating for substance Heating element may get in the way of mixer High watt density Risks Cheap, easy to order custom, Kapton coating Clamping system required. Best used for conduction heating Requires tight tolerances for placement Small sizes don’t have high wattages Type Heating application Typical off the shelf example – selected for size then power Power of example Watt density of example (W/in2) Internal Resistance for example ( ) Current at 12V (amp) Overall Size of example (inches) 5.1.5.2.0 Strip Gases or solid surfaces Omega PT512/120 2.5W at 12V Band Solids in cylindrical form Omega MBH1215200A /120 Chemical Heaters Chemical heaters use a chemical change to produce heat in an exothermal reaction. This could be done by adding water to some chemical or mechanically activating the chemical change. An example of this type of heating is an over the counter hot pack. An advantage of this option is that they do not use electrical power. Disadvantages are that they are complex if autonomous, needs a supply of water or mechanical mechanism to initiate chemical change, require a supply of materials be restocked after use and are difficult to control temperature. This last disadvantage greatly lowers the feasibility of using option 2, based on our strict temperature control requirement 4.2. Page 45 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.5.3.0 MiDAs December 18th, 2006 Solar Exposure The last option utilizes heat from direct solar exposure. One method would be to use the sun to provide direct heating through the use of a solar oven configuration. An advantage of this method is that it does not use electrical heating. The disadvantages are the complexity to focus energy where needed, its difficulty to control and that the sun does not always shine. 5.1.5.4.0 Conclusion The determination was made to use internal resistance heaters. They will be the best solution to heat the environment in the worst case scenario using the limited power supply of 30W. They also allow for an interface with a control system to stay within the range of ±1°C. 5.1.6.0 Cooling Element Trade Study A trade study to determine a cooling element was conducted. This was based on requirement 4.2. The temperature control of ±1°C will be delivered through the use of a control system, not the cooling element. The temperature could exceed 370C in the Atacama Valley in Chile stated in requirement 4.30. There were two options for cooling shown below. 1. Passive cooling. 2. Cooling through the use of a heat switch. 3. Active cooling through TECs, which are solid state heat pumps. 5.1.6.1.0 Passive Cooling Radiation and conduction on the outside walls of the chamber were used in the thermal analysis to create cooling curves to determining if passive cooling was feasible. The advantages of passive cooling are that it does not use electrical cooling and it’s very simple. Its disadvantages are that the geometry will determine if there is enough space available for this to work and it will take a long time. This is unfeasible due to the fact the ambient temperature could exceed the bounds of requirement 4.2. 5.1.6.2.0 Heat Switch Cooling through the use of a heat switch is another option. A heat switch is a device that changes thermal conductivity with varying temperature. A substance melts inside the switch that would then allow two internal plates to come into contact, increasing thermal conductivity. A heat switch could be attached to a heat sink that would increase the effective Page 46 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 surface area available for cooling when needed. An example of a heat switch is the Starsys Research Diaphragm Thin Plate switch. A 0.13mm gap separates the hot and cold sides. A paraffin layer melts at a prescribed temperature increasing in volume by over 15%. This pushes a conducting plate across the gap, increasing the thermal conductance. The thermal conductance ratio between open and closed configurations is 92:1. The available melting temperature range for the paraffin is -950C to +860C. Advantages of this option would be that it does not use electrical cooling, allows most sides of chamber to be insulated while still allowing a surface to be available for cooling and its space flight tested – currently used on MER for thermal regulation on batteries. The disadvantages are its more complex implementation, cost, availability and technical data is unavailable. 5.1.6.3.0 TEC Active cooling through TECs was another option. TECs are solid state heat pumps. An example of a typical TEC is a Melcor CP1.0-127-05L-1-W5. This has a maximum temperature differential between the two sides of the device of 670C when a max voltage of 15.4V is applied. This results in a max current of 3.9 Amps. The total cooling at these maximums is 33.4W. It is very small at 1.18” x 1.18” x 0.13” (30mm x 30mm x 3.2mm) and weighs 0.024lbs (0.011) and costs $15.54. Custom TECs are available that cascade individual units to produce a larger maximum temperature differential. Their advantages are their concentrated cooling power and that most sides are allowed to be insulated while allowing a surface to be available for cooling. The disadvantage of this option is the use of electrical power. 5.1.6.4.0 Conclusion The ultimate decision to use TECs was based on their ability to satisfy requirement 4.2. They will be able to heat and cool the environmental chamber within the temperature requirements. For greater heating capability strip heaters will supplement the TECs. 5.1.7.0 Insulation Trade Study The decision to use insulation was based on requirement 4.24. Insulation minimizes the amount of power needed to increase the temperature of the reaction chamber and thus meeting requirement 4.24. A thermal analysis was conducted to determine the amount and type of insulation needed. A trade study on insulation was conducted and three options were determined. These options exclude insulations that are unable to reach a minimum of 1400C to come in contact with the TECs. 1. Pyrogel 6250. 2. Melamine foam sheets. 3. Fiberglass. Page 47 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 5-3 shows the Thermal properties of each insulation option. Pyrogel Melamine Fiberglass 5.1.7.1.0 Table 5-3 Insulation Options Thermal Cond. Min. Temp. (W/m-K) (Celsius) 0.0155 -200 0.53 -50 0.0576 -97.8 Max. Temp. (Celsius) 350 150 232 Pyrogel 6250 Pyrogel 6250 has a very low Thermal Conductivity or k value. It is also a member of the Aerogel family and has been flight tested. It is easy to machine and can be cut with scissors. The only disadvantage with this option is its large cost. We would have to order a minimum roll of 4.66’ x 30.0’ (1.42m x 9.14m) with a thickness of 0.24” (6mm). This comes at a cost of $776. Based on the suggestion of the PAB we concluded that Pyrogel is overkill and decided to go with another option. 5.1.7.2.0 Melamine Melamine has a higher k value, but this can be overcome by increasing the thickness of the insulation. A great benefit is that the cost is much lower than that of Pyrogel 6250. It is easy to machine and can be cut with scissors. We will also receive much more than we can use to ensure duplication if there is a problem with insulation attachment. A thermal analysis was conducted and determined that the time would be feasible based on a half inch thickness along the walls of the reaction chamber’s environment. 5.1.7.3.0 Fiberglass Fiberglass has a lower k value than Melamine and is very cheap. A roll of fiberglass insulation was purchased to use for the autoclave prototyping. This was probably enough for the entire apparatus, but there were concerns about machining the fiberglass. When you cut fiberglass small particles waft into the air and are a safety hazard. This was a great disadvantage and the machinist conducting the autoclave prototyping test determined not to use fiberglass for insulation applications. 5.1.7.4.0 Conclusion The decision was made to use Melamine to insulate the environmental chamber enclosure. This was based on the facts that Pyrogel 6250 was deemed overkill for this project and that fiberglass, while inexpensive, carries safety hazards while machine. Page 48 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.8.0 MiDAs December 18th, 2006 Mixing Trade Study The decision to mix the solution was based on requirement 4.5. The electrochemical sensors need mixing to successfully monitor the sample. There are three options to consider for mixing. 1. Ultrasonic 2. Magnetic 3. Mechanical 5.1.8.1.0 Ultrasonic The ultrasonic processor of choice is the Sonaer Ultrasonics P/N S900PIEZO shown in Figure 5-1. The magnetic stirrer is yet to be determined and the mechanical mixer will be a prototype that was previously tested in space applications. Figure 5-1 Sonaer S900PIEZO Ultrasonic Processor This option was unfeasible due to the large size of the mixer. We need this to fit inside the small reaction chamber at a diameter and length much smaller than the smallest mixers available to us at a reasonable cost. 5.1.8.2.0 Magnetic A magnetic mixer is the second option. There is a pending test by Tufts University to determine whether or not a magnetic mixer will affect the ISEs. If a magnetic mixer does affect the ISEs that will immediately eliminate that option. 5.1.8.3.0 Mechanical The third option was a mechanical mixer. A prototype was created and tested with various types and granularities of samples with water. The test was performed visually to evaluate the fluid flow and to observe any sedimentation that may be present. The test had some problems, but was able to achieve the desired results. Page 49 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.8.4.0 MiDAs December 18th, 2006 Conclusion The end result was that the prototype functioned to the desired result. This verified our preliminary decision to use mechanical mixers. This will be the choice until we hear positive feedback from the test at Tufts University. If this information is received then we will reevaluate the effect of a magnetic mixer and implement this in the design if need be. 5.1.9.0 Temperature Sensor Trade Study The MiDAs project will utilize temperature sensors to verify requirements 4.2. This verification will be achieved by placing each type of sensor inside the reaction chamber environment. There are three options for Temperature sensors shown below. 1. Thermocouples 2. Thermistors 3. RTDs 5.1.9.1.0 Thermocouples The advantages of using thermocouples are a variety of types and configurations, low cost, widely available, rugged, thoroughly tested, self-powered and do not self-heat. Their disadvantages are that they require a CJC for calibration and the sensor accuracy can reach 1°C at temperatures between 10°C and 40°C. 5.1.9.2.0 Thermistors The advantage of using Thermistors is that they are sensitive (typically have better accuracy then Thermocouples and RTDs). Their disadvantages are that they have a loss of linearity and temperature curves have not been standardized to the extent of Thermocouples or RTDs. Thermistors also require shielding from large temperatures and a current to take measurements resulting in self-heating. These disadvantages greatly increase complexity. 5.1.9.3.0 RTDs The advantages of using RTDs are their high accuracy, excellent stability and reusability; they can be immune to electrical noise, they require shielding from large temperatures, require a current to take measurements which plagues it to self-heating. These options are shown next to each other for comparison in Table 5-4. Page 50 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 5-4 Temperature Sensor Comparison Thermocouples Model Cost Volume Temp Operation Accuracy Range Range (1-40 °C) Weight Diameter Length 5SRTC-TT $54 (5 (mini connector) pack) --- 0.51mm 1m T --- ±1 °C 5LRTC-TT (standard size) $58 (5 pack) --- 0.51mm 1m T --- ±1 °C 5TC-TT (no connector) $39 (5 pack) --- 0.51mm 1m T --- ±1 °C $92 --- 1.6mm 1m JKTE --- --- TJ36 (autoclave probe) Thermistors 44005 $15 T (Copper) -200 to 350 °C --- RTD -80 to 250 °C 2.8 60 Height Length Width --- ±1 °C Self heating SA1-RTD $50 --- 25 2m 19 -73 to 260 °C --- ±0.5 °C FR1328 $15 --- 6 22 2.8 -70 to 500 °C --- ±0.2 °C 5.1.9.4.0 Conclusion In conclusion the general purpose model FR1328 should work well in terms of price and accuracy. Therefore we chose to use a RTD for the temperature measurement in the reaction chambers. 5.1.10.0 Pressure Sensor Trade Study The MiDAs project will utilize the use of pressure sensors to satisfy requirements 4.3. There are three options for pressure sensors. 1. Gauge 2. Absolute 3. Differential Page 51 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.10.1.0 MiDAs December 18th, 2006 Gauge The advantages of using gauge sensors are that they are widely available and relatively cheap. Their disadvantage is that they measure the pressure relative to standard atmosphere. 5.1.10.2.0 Absolute The advantages of using absolute sensors are that they measure pressure relative to vacuum, are widely available and relatively cheap. Their disadvantage is that they require additional sensors to measure local atmospheric conditions. 5.1.10.3.0 Differential The advantages of using differential sensors are their ability to measure the difference between two locations. Their disadvantage is that they are slightly more expensive then gauge and absolute sensors. A comparison of the pressure sensors is shown in Table 5-5. Table 5-5 Pressure Sensor Comparison Model Cost Wetted diameter Height Length Width Temp Range Power Accuracy *** Threaded PX138 * $85 --- 26.2 28.1 27.9 0 to 50 °C 8VDC 0.1% 0.5% No PX139 * $85 --- 26.2 28.1 27.9 0 to 50 °C 5VDC @2 mA 0.1% 0.5% No PX140 * $120 --- 26.2 28.1 27.9 -18 to 63 °C 8VDC 0.75 % 0.15% No Diameter Length 12 57.9 -54 to 121 °C 24VDC @15 mA 0.25% 0.25% Yes PX209 ** $195 13 * gage, absolute and differential available ** gage and absolute available *** Linearity/Hysteresis Note: all parts available in Gage, Absolute and Differential. Dimensions given in mm. Page 52 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.1.10.4.0 MiDAs December 18th, 2006 Conclusion Pressure sensors for the reaction chambers will not be required to maintain any seal and therefore the Model PX139 is recommended for its low power usage. Therefore we will utilize the gauge sensor option to lower cost and have a wealth of information in respect to their performance. 5.2.0 Autoclave 5.2.1.0 Subsystem Design-to Requirements Requirement 4.11. The reaction samples shall be sterilized in accordance with standard autoclave techniques. Requirement 4.24. MiDAs shall provide its own power (between 10 W and 30 W) in a field setting. Requirement 4.26. Nominal power consumption shall not exceed 30 W. Requirement 4.27. Peak power consumption shall not exceed 30 W for more than 30 seconds. 5.2.2.0 Autoclave Techniques Standard autoclave techniques are to heat the sample to 1210C and hold it at that temperature for 15min. This will kill the organisms that are unable to put up their protection. After the 15min. time period is finished the autoclave will cool to 300C for a period of 24 hours. At this time the organisms will be given the chance to remove their protection and a second autoclave cycle will begin. Three cycles will occur before the samples area sterilized and they consist of heating the autoclave, holding it, cooling it back down and holding it at a stable temperature of 300C. This process cannot exceed 30W. This is based on requirements 4.24, 4.26 and 4.27. 5.2.3.0 Thermal Analysis Now that the specific requirements were taken into account a thermal analysis was conducted to determine the feasibility of creating a chamber and running one autoclave cycle. This analysis is based on looking at how much internal energy change is required to raise or lower chamber temperature from -100 C to 1210C which is the required temperature for sterilization. The various stages of the sterilization process will each have a different temperature range and calculations for each will be shown in following sections. In general, knowing the required internal energy change, the amount of energy available that can be input into the system and the amount of energy that is leaving the system, it is possible to Page 53 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 calculate the time required to complete the change. The Internal energy change of a chamber for 316 SS and the contents as a water/air mixture is shown in equations 5-1 and 5-2. U sys U steel U water Q (5-1) Q [( m)(c p )(T2 T1 )] steel [( m)(c p )(T2 T1 )] water (5-2) The contents of a chamber will be a 1.53in3 (25cm3) sample (modeled as water here), 0.305in3 (5.0cm3) of water (1/2 of which will get vaporized to provide steam for the sterilization) and 1.70in3 (27.92cm3) of empty space. The mass of the contents composed of soil (modeled as water), water, and space is 0.13lb (0.058kg). The cp for 316 steel is 452J/kgK while the cp for water is 4,230 J/kg-K. The temperature difference requires vaporizing half the 5cm3 of water for steam. This will require the heater to supply the energy needed for the latent heat of vaporization that the phase change requires. The latent heat of vaporization for water is 2,257KJ/kg. This will be undertaken in five stages. 5.2.3.1.0 Stage 1 The sample is heated from a worst case ambient temperature of -100C to 1210C. This stage requires some of the water added to the sample to be vaporized and the energy for this is included in the calculations. The heating requirements based on these temperatures require 52.74KJ. 5.2.3.2.0 Stage 2 The sample is hold at 1210C for 15min. The energy required for this stage will be equal to the energy lost during this time. 5.2.3.3.0 Stage 3 The sample is cooled to 200C. The water that was vaporized in stage one will condense in this stage and the energy lost from the system is included in the calculations. The cooling requirements for temperatures of 1210C and a lower temp of 200C is an energy change of 30.67KJ. 5.2.3.4.0 Stage 4 The sample is held for 24 hours at 200C. The energy required for this stage will be equal to the energy lost during this time. Page 54 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.2.3.5.0 MiDAs December 18th, 2006 Stage 5 The sample is heated from the lower holding temperature back to the sterilization temperature. The heating requires upper temperature of 1210C and a lower temp of 200C returning an energy change of 41.96KJ. To complete the sterilization phase, stages 2-5 will need to be completed again followed by another holding and cooling time represented by stages 2-3. 5.2.3.6.0 Conclusion The heater and cooler will transfer energy in and out of the system. A TEC operating as a heat pump was chosen to allow one piece of hardware to perform both functions. It was calculated that the data system would require 6W of power during the sterilization phase of the project, leaving a max of 24W available for heating and cooling. The overall system would require 2 chambers and each one provides samples to the reagent chambers following sterilization. 24W is available for the heaters and coolers modeling a staggered heating cycle. It was found that the TEC considered has an efficiency of 56% so that only 13.44W of energy was available for an energy change to the system from the heater/cooler. A thermal resistance network was used to model the steady state heat loss through the insulation surrounding the chambers. 5.2.4.0 Insulation The same insulation that was purchased and used for the reaction chamber environment will be used for the autoclave. This Melamine insulation was used in the prototyping of the autoclave and led to a positive result and the acquisition of the 1210C mark. The decision to use Melamine is shown in section 5.1.7. 5.2.5.0 Material Selection The material selection was important in the determination of how to safely seal the autoclave which is a pressurized chamber. The material for the autoclave must be able to withstand temperatures of 1400C and a maximum pressure of 35psi (241KPa). The autoclave chamber material must also be highly corrosion resistant. Soils release salts when mixed with water and heated, and the chamber must be able to withstand that corrosion. Section 5.1.4 details some of the wetted materials available for MiDAs based on their biocompatibility and autoclavable. Based upon the strong anti-corrosive properties and ability to withstand high temperatures it will be used for the construction of the autoclave. Page 55 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.2.6.0 MiDAs December 18th, 2006 Heating and Cooling Apparatus To determine what heating and cooling element needs to be used we looked at a variety of options shown in section 5.1.4. Using the knowledge from this trade study TECs or passive cooling must be chosen to feasibly cool the autoclave. A thermal analysis was conducted using Melamine as our insulation and passive cooling was ruled out due to the large amount of time it would take to cool (see Chapter 8 for thermal analysis). This made the decision for us to choose TECs as our cooling option. Therefore we will use the TECs as our heating option also. Upon further thermal analysis and prototyping (see Chapter 7 for prototyping results) the solution to heat the side that is not in contact with the TEC to the 1210C temperature was to use three strip heaters. These will be used in conjunction with the TEC and allow the internal volume of the autoclave to reach the desired temperature for 15min. 5.2.7.0 Sensors A trade study on temperature sensors was completed and a sensor was chosen based on the information in section 5.1.9. For autoclave purposes Model FR1328 was chosen for its accuracy and low cost (an additional Swagelok (omegalok) adapter is required for sealing the autoclave). To determine the pressure sensors the trade study in section 5.1.10 was used. For the autoclave, a gauge sensor will provide the necessary pressure data and will generally cost the least. The autoclave will be pressurized through the use of a threaded connector creating a tight seal. The Model PX209 is recommended for use to ensure this pressurization. The small wetted diameter (13mm) is ideal for fitting an autoclave chamber if need be. 5.3.0 Sample Transportation 5.3.1.0 Requirements Sample transportation is the act of moving the samples from the autoclave chambers to the Reaction Chambers. This will be accomplished through the use of a tube design. Trade studies were conducted to determine the tubing material and valve structure. Requirement 4.7. Each reaction chamber shall be manufactured out of a list of materials provided by BioServe. This list includes, but is not yet limited to, Polysulfone, PharMed, 316 stainless steel, and Ultem 1000. Requirement 4.8. Each reaction chamber shall receive no less than 5 mL and no more than 25 mL of geological sample. Requirement 4.15. The sterile reagent water shall be completely contained in both solid and liquid form. Requirement 4.16. The MiDAs shall aseptically deliver no more than 50 mL (within ± 5% accuracy) of sterile reagent water to each reaction chamber. Page 56 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 5.3.2.0 MiDAs December 18th, 2006 Material Selection Based on requirement 4.7 biocompatible, materials must be used for the wetted surfaces. To determine the material section 5.1.4 and the materials trade study was utilized. For the soil and water transport, PharMed is an excellent choice due to its wide operating temperature range and availability of sizes. 5.3.3.0 Valve Selection There is a problem with the amount of the sample trapped between the autoclave and the valve. This section of the sample runs the risk of not being autoclaved during its cycles which will disrupt the results of the experiment. Another belief is that too much heat will be lost through the metal valve and that a ceramic valve should be used. For these reasons it was determined that the preliminary selection of a ball valve would not satisfy requirement 4.11. Other options were selected and are listed below. 1. Ceramic valves 2. Ductile iron butterfly valves shown in figure 5-2 Figure 5-2 Butterfly Valves 3. Bronze needle valves shown in Figure 5-3, below. Page 57 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 5-3 Needle Valve 5.3.3.1.0 Ceramic Valves Theoretically we could construct a ceramic valve. The problem with that is pressurizing a machined valve brings up safety concerns. It would not lose much heat, but due to the fact that ceramic is very brittle this will be too tough to machine and is unfeasible. 5.3.3.2.0 Butterfly Valve To decrease the surface area in contact with the autoclave we should use a butterfly valve. This valve satisfies the pressure and temperature requirements stated in our PDD while minimizing the surface area conduction will flow through. The valve can also be operated electronically. The design of a butterfly valve ensures that the sample is very close to the bottom of the autoclave. This means that the sample with be thoroughly heated and the Autoclaving cycles will sterilize the entire sample. This was the main problem with the valve selection and this design will satisfy this criteria. 5.3.3.3.0 Bronze Needle Valve The bronze needle valve will have to be welded onto the autoclave at the bottom. This would mean that the tubing will be used with the valve to move the sample. This leaves a small but unsatisfactory portion of the sample in the tubing. This small part will not be autoclaved and could skew the results of the experiment. 5.3.3.4.0 Conclusion The end result is that a butterfly valve was selected. This was ordered and taken apart to ensure that the sample is as close to the autoclave as possible. It will be screwed onto the autoclave and therefore be able to be removed to be sterilized separately after each experimental run. Page 58 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 6.0 Subsystem Design-To Specifications Author: Ted Schumacher Page 59 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 6.1.0 Overall System Architecture The MiDAs project is composed of 48 parts that require drawings either from the manufacturer or the MiDAs team. The overall system architecture is shown below in figure 6-1. Figure 6-1 Overall System Architecture Here you can see the Autoclaves at the top in lavender, the peristaltic pump in the back in green, the reagent water chamber next to the pump in silver, the sample transportation medium under the Autoclaves in yellow, the reaction chamber environment in tan, reaction chambers in orange and chassis in white. The power system is not shown due to its limited priority in the overall project. This is due to the fact that we are creating a prototype based on a theoretical Mars mission. Therefore we can assume we will be receiving power from a “Mars Rover” when in actuality this will be strictly an Earth laboratory and field instrument. The power supply will likely come from a wall outlet or a generator. After the trade-studies were completed each specific subsystem was designed to decrease complexity, usually in the Page 60 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 form of mechanical parts. By minimizing the amount of stationary and moving parts we limit complexity for the overall project. The major subsystems and their corresponding components will need to be integrated into the final project. A top level integration plan was created to understand the overall picture and the incorporation of each individual subsystem in the scope of the overall project. The integration plan is shown in figure 6-2. The integration plan is explained further in Chapter 11. Figure 6-2 Top Level Integration Plan As you can see the main structure or chassis is connected to all the main elements of the project. This flows down from that point onto how each specific subsystem will be assembled and the parts that make up that assembly. This integration plan breaks down the work that needs to be completed for the project to be a success. To understand the entire scope of the project and the vast amount of testing required an experimental timeline was constructed and is shown in figure 6-3. Page 61 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis Minutes Description t=0 Begin autoclave Measurement temperature Test Is data being recorded? pressure t=1 to 120 approx. t = 121 to 135 Heater is on temperature Cooler is off pressure Temperature control above 121°C temperature Heater is variable pressure Cooler is off t=136 to 256 approx t = 257 to 317 temperature Heater is off Cooler is on pressure Temperature control between 437°C for 1 hour temperature Yes/No Control yes -------------- no Is temperature >= 125°C? Is t =135? Is temperature <= 123°C? Begin cooling MiDAs December 18th, 2006 Is temperature <= 35°C Is t =317? pressure Is temperature <= 6°C? Is temperature >=35°C? yes Stop, check for failure -------------- no heater cooler on ----- increment time, move to next step ----- ----- stop off ----- -------------- on ----- yes -------------- off on no -------------- ----- ----- yes -------------- on ----- no -------------- off ----- yes -------------- ----- off no -------------- ----- on yes -------------- on off no -------------- ----- ----- yes -------------- on off no -------------- off off yes -------------- off on no -------------- off off increment time, move to next step increment time, repeat until >= 125°C increment time, move to next step perform next test increment time, repeat until t = 135 increment time, repeat until t = 135 increment time, repeat until <= 37°C increment time, move to next step increment time, move to next step perform next test perform next test perform next test increment time, repeat until t = 317 increment Page 62 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 time, repeat until t = 317 t = 318 t = 636 t = 954 begin autoclave cycle again begin autoclave cycle again Autoclave procedure complete Is t = 953? visual Are heater and cooler off? yes off off no ----- ----- yes no Heater is off Cooler is off t = 955 t = 956 begin soil transportation finish soil transportation Are valves open? yes no temperature initialize sensors pressure inoculate Electrochemical begin mixing temperature temperature control pressure Electrochemical Is pump done? Is data being recorded/electrochemical zeroed? Is test chamber inoculated? no Stop, check for failure close valves, begin taking measurements, zero electrochemical sensors -------------- yes inoculate no Stop, check for failure turn on mixers yes yes no Is temperature <= 6°C? Is temperature >=35°C? open auto clave ejection valve and water valve Stop, check for failure pump 25mL water through autoclave increment time, move to next step continue autoclave cycle perform next test stop increment time, move to next step stop perform next test increment time, repeat increment time, move to next step stop perform next test yes stop, check experiment for failure -------------- stop on off no -------------- off off yes -------------- off on perform next test perform next test increment time, repeat until t = 21116 Page 63 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 no -------------- off off increment time, repeat until t = 21116 Figure 6-3 Experiment Timeline Now that the amount of testing is determined the work entailed in the entire scope of the project will be able to be understood. Here the testing can begin when the first Autoclave, sample transport and reaction chamber and assembled. This gives us the opportunity to begin testing early in the Spring semester of 2007 and continue it on to the end of the semester. The work breakdown structure was devised from Figures 6-2 and 6-3. There are five subsystems that need to be integrated with the Chassis. These are the Autoclaves, reaction chamber environment, reagent water chamber, DAQ and controls and the power supply. These require different levels of expertise and vastly different amounts of work load. 6.1.1.1.0 Autoclave The Autoclave requires a cap, temperature and pressure sensors and screws for the cap attachment. These will be interfaced with the cap. The body of the Autoclave utilizes an Oring to pressurize the vessel along with TECs and three strip heaters for heating and cooling. A heat sink will also be attached to the rear of each TEC. The autoclaves will be machined first to begin testing as soon as possible. This will require the largest allocation of resources and when it is completed then the testing schedule can begin. This will give the group the opportunity to autoclave the soil and determine if sample transport out of the autoclave will be feasible with the current design. 6.1.1.2.0 Environmental Chamber The reaction chamber environment houses a pressure sensor along with TECs. This will be one of the last pieces to be assembled. The testing required will be to heat the environment with the two reaction chambers inside. A 1psi pressure differential is needed along with controlling the temperature based on requirement 4.2. The temperature sensors will be housed in the reaction chambers to ensure the samples are at the exact temperature stated in requirement 4.2. The reason the environmental chamber was chosen was to eliminate disparity in the temperature control in both of the reaction chambers. The construction is not too intensive and it will be able to be pushed back if need be. 6.1.1.3.0 Reaction Chambers The reaction chambers are inside the shared environmental chamber. These are composed of the chamber body assembly, body and environmental sensor package defined in requirement 4.4. There will be an interface in the chamber for the environmental sensors as well as a temperature sensor and multi-use ports defined in requirement 4.6. The mixing apparatus will be located in the bottom of each reaction chamber. The mixing trade-study is shown in section 5.6.1. Here a mechanical mixer was chosen that requires a motor, bearing, gears and an impeller. This system will be tested at first with the autoclave to determine if the sample Page 64 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 can be transferred to it successfully. Therefore this chamber will be the second part machined and be able to continue the rigorous testing schedule after the autoclave’s assembly. 6.1.1.4.0 Reagent Water Chamber The reagent water chamber is needed to more the water to the autoclaves flushing the sample down to the reaction chambers. This will be accomplished through the use of two peristaltic pumps attached to two autoclaves. The system will require the machining of the chamber, a temperature sensor and a strip-heater. This system is low on the priority list and will be the last chamber to be machined. 6.1.1.5.0 Data Acquisition The DAQ system requires an embedded CPU and an interface for DAQ. This will include all of the 10 sensors needed for the project and satisfy the need for an interface connecting the controls to the sensors. 6.1.1.6.0 Power Supply The power supply will be modeled as a wall outlet and interface to the system. 6.1.1.7.0 Chassis The Chassis will require little machining and will be the last part to be created. This will consist of the body and some type of transportation interface to make it a field unit. The determination at this time is using handles, but this can change based on customer preference. Everything will be completed at least one week before the last machining day. This will give us time for the whole integration. Integration and testing will begin as soon as the first part is created, but we need to ensure a week lag time for any problems that might occur in the final attachment of the entire system to the Chassis. 6.1.1.8.0 Organizational Chart A flow-chart of the organizational chart is shown in figure 6-4. This details the specifics of who will be working on each sub-system in the Spring Semester of 2007. The organizational chart is explained further in Chapter 13. Page 65 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 MiDAs Project Management Fabrication Design Document Verification and Testing Project Manager Elizabeth Newton Lead Fabrication Engineer Dave Miller Design Engineer Chuck Vaughan Systems Engineer Shayla Stewart Assistant Project Manager Ted Schumacher Assistant Fabrication Engineer Sameera Wijesinghe Design Engineer Jeff Childers Software Engineer Steven To Assistance as Needed from Team Assistance as Needed from Team Assistance as Needed from Team Figure 6-4 Organizational Chart 6.2.0 Subsystem Design 6.2.1.0 Chassis Design The Chassis component will be assembled using Aluminum. This is due to the inexpensiveness of Al and its low relative density. Requirement 4.7 states: Each reaction chamber shall be manufactured out of a list of materials provided by BioServe. This list includes, but is not yet limited to, Polysulfone, PharMed, 316 stainless steel, and Ultem 1000. This refers to the wetted materials of the project. The Chassis will not come into contact with the sample and therefore is not beholden to requirement 4.7. The Chassis is made up of two plates that are 6.0” x 4.0” with a thickness of ¼” (15.0cm x 0.64cm x 0.16cm). These plates will be attached by Al L-shaped brackets at each of their four ends. These brackets have dimensions of 4.0” x 0.5” x ¼” (10.2cm x 1.27cm x 0.64cm). The mass for the entire Chassis is 1.15Kg. The Chassis will be assembled using screws attached to the L-shaped brackets and the plates. The Autoclaves, reagent water chamber, reaction chamber environment, DAQ and control system and power supply will all be attached to the Chassis. 6.2.2.0 Autoclave Design The Autoclaves will be constructed of 316 Stainless Steel. This determination was shown in section 5.1.3.1. Through a mass analysis based on the dimensions from the Autoclave Page 66 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 prototype in Chapter 8 the mass of the Autoclaves is 4.17lbs (1.89Kg). Melamine insulation was chosen to house the Autoclaves as described in section 5.1.5. There is a 2” diameter to the insulation which is a mass of 0.0217lbs (9.83g). The TECs will be attached to the flat side of the Autoclaves. The two TECs have a combined mass of 0.794lbs (360g). Therefore the entire Autoclave system (Autoclaves, TECs and insulation) has a total mass of 4.99lbs (2.26Kg). 6.2.3.0 Reagent Water Delivery Design The reagent water system is made up of the peristaltic pumps, the reagent water chamber, and the tubing required to transfer the fluid to the autoclaves. The peristaltic pumps are purchased and weight 0.140lbs (63.5g). The reagent water chamber will be designed with the dimensions stated in Chapter 8 and has a mass of 0.291lbs (132g). The tubing required to transfer the reagent water has a small diameter. This relates to a mass of 0.026lbs (12g). Therefore the total mass of the reagent water system is 0.457lbs (208g). 6.2.4.0 Environmental Design The reaction chamber environmental system is composed of the reaction chambers, insulation, TECs and mixers. The reaction chamber environment is Al because it is not beholden to requirement 4.7. It has the dimensions of 6.80” x 4.70” x 3.92” (17.28cm x 11.95cm x 9.95cm). Based on a 1/8” (0.5cm) thickness for the Al walls, the mass of the chamber is 1.45lbs (656g). The insulation covers the outside of the chamber and has a mass of 0.0214lbs (9.72g). The TECs have a mass of 0.794lbs (360g) and the mixers have a mass of 0.21lbs (95.8g). Therefore the total mass of the reaction chamber environmental system is 2.47lbs (1.12Kg). 6.2.5.0 Data Acquisition Design The DAQ system and control system are composed of all the sensors, power supply interface, embedded CPU and the environmental sensor package. We are using a temperature and pressure sensor to measure the ambient conditions outside the MiDAs apparatus. A pressure and temperature sensor will be used to measure the conditions inside the Autoclaves. Temperature sensors will be inside the reaction chambers but there will only be one pressure sensor in the reaction chamber environment. There will be a temperature sensor inside the reagent water chamber to ensure liquid conditions. There are a total of 6 temperature sensors and 4 pressure sensors. Along with the embedded CPU and the DAQ the total mass is 0.64lbs (292g). 6.2.6.0 Mass Overall the total mass stands at 30lbs (13.9Kg) and can successfully be transported and used as a field instrument. A diagram of the mass analysis is shown in figure 6-5. Page 67 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 2 Pumps 127g Insulation 9.83g Water Chamber 132g 2 Autoclaves 1890g 2 Valves 1450g 4 TECs 720g Environmental Chamber 656g 2 Reaction Chambers 173g Chassis 1150g 2 Mixers 95.8g CPU and DAq (not shown) 292g Internal Mass: 7.10kg (15 lbs) Total Mass: 13.90 kg (30 lbs) Sensors (not shown) 10.0g Figure 6-5 Mass Analysis 6.3.0 Power requirements 6.3.1.0 Requirements The power requirements are 4.24, 4.25, 4.26 and 4.27. The power consumption for the autoclave cycle is listed below. The numbers show the steps in the autoclave process. 1. 5.5 W continuous power consumption by Data Acquisition System (DAQ) 2. 0.5 W continuous power consumption by temperature and pressure sensors 3. 12 W per autoclave heater/cooler (2 autoclaves) 4. Temperature of autoclaves begins at -10°C (worst-case) 5. Time to heat from -10°C to 121°C is 234 min (see thermal analysis) 6. Hold at 121°C for 15 min – consumes 1.4 W 7. Time to cool from 121°C to 20°C is 117 min (see thermal analysis) 8. Hold at 20°C for 24 hrs (1440 min) – consumes 1.4 W 9. Time to heat from 20°C to 121°C is 205 min (see thermal analysis) Figure 6-6 shows the power summary for the entire sterilization phase of the MiDAs operational cycle for simultaneous autoclaves. This is based off the nine steps that use power in autoclave cycle. The autoclaving process must be cycled three times to ensure sterility of the soil. The maximum power consumption is the maximum allowable at 30W, with no buffer built in. Page 68 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Power Summary (Sterilization Phase) Power Consumption (W) 35 30 25 Autoclave Heater/Cooler 1 Autoclave Heater/Cooler 2 DAq Temp/Pressure Sensors Total Power 20 15 10 5 0 30 0 60 0 90 0 12 00 15 00 18 00 21 00 24 00 27 00 30 00 33 00 36 00 39 00 0 Operation Time (min) Figure 6-6 Power Summary (Full Sterilization Phase) The entire sterilization phase takes 3,930mins (65.5 hrs or 2.73 days). A summary of the power used after each section of the experiment is started. Heat to 121 C Hold at 121 C Cool to 20 C Hold at 20 C Heat to 121 C Hold at 121 C Cool to 20 C Hold at 20 C Heat to 121 C Hold at 121 C Cool to 20 C Table 6-1 Power Summary Start Time Duration (min) (min) 0 234 235 15 251 117 369 1440 1810 205 2016 15 2032 117 2150 1440 3591 205 3797 15 3813 117 Stop Time (min) 234 250 368 1809 2015 2031 2149 3590 3796 3812 3930 Due to figure 6-6 to ensure that peak power consumption is minimized the autoclaves will be staggered. Figure 6-7 shows the power summary for the entire sterilization phase of the MiDAs operational cycle for staggered autoclaves. Staggering the autoclaves allows each autoclave heater/cooler to run on higher power (20W compared to 12W). The autoclaving process must be cycled three times to ensure sterility of the sample. The maximum power consumption is 26W, which gives a 4W buffer. Page 69 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Power Summary (Sterilization Phase) Power Consumption (W) 30 25 Autoclave Heater/Cooler 1 Autoclave Heater/Cooler 2 DAq Temp/Pressure Sensors Total Power 20 15 10 5 0 30 0 60 0 90 0 12 00 15 00 18 00 21 00 24 00 27 00 30 00 33 00 36 00 39 00 0 Operation Time (min) Figure 6-7 Power Summary (Sterilization Phase) - Staggered Autoclaves The entire sterilization phase takes 3,307mins (55.1 hrs or 2.30 days). Table 6-2 Power Summary (1st Autoclave) Start Time Duration Stop Time (min) (min) (min) Heat to 121 C 0 90 90 Hold at 121 C 91 15 106 Cool to 20 C 107 46 153 Hold at 20 C 154 1440 1594 Heat to 121 C 1595 72 1667 Hold at 121 C 1668 15 1683 Cool to 20 C 1684 46 1730 Hold at 20 C 1731 1440 3171 Heat to 121 C 3172 72 3244 Hold at 121 C 3245 15 3260 Cool to 20 C 3261 46 3307 Table 6-3 Power Summary (2nd Autoclave) Start Time Duration Stop Time (min) (min) (min) Heat to 121 C 154 90 244 Hold at 121 C 245 15 260 Cool to 20 C 261 46 307 Hold at 20 C 308 1440 1748 Heat to 121 C 1749 72 1821 Hold at 121 C 1822 15 1837 Cool to 20 C 1838 46 1884 Hold at 20 C 1885 1440 3325 Heat to 121 C 3326 72 3398 Hold at 121 C 3399 15 3414 Cool to 20 C 3415 46 3461 Page 70 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 6-7 as well as Tables 6-2 and 6-3 show that staggering the autoclaves is beneficial in more than respect. The autoclave heaters/coolers can run on higher power, which reduces the time to heat and cool to the desired temperatures. The entire sterilization process then takes 55.1 hrs instead of the 65.5 hrs that the simultaneous autoclaving requires. The maximum power consumption for sterilization is also reduced to 26 W which leaves buffer for the maximum power requirement. 6.3.2.0 Power The power requirements are 4.24, 4.25, 4.26 and 4.27. These must be followed to ensure the sample remains at the correct temperatures while in the reaction chambers. These require power for heating and cooling. It was calculated elsewhere that during the testing phase, electronics and mixers may require 20W of power. This leaves 10W for the thermal control of the environmental box. Using the geometry defined above with a 10W rated heater/cooler that is capable of supplying 5.6W of energy (only 56% efficient) and insulation allowing only 1.55W for a worst case energy loss, a power and time model for the overall sterilization phase was constructed. A 10W heater/cooler will drawn 0.83 amps (P=VI) at 12V. At 12V, a 1.55W heater or cooler will draw 0.129 amps. This analysis was used for initial and maintenance cycles. 6.3.2.1.0 Heating The initial heating problem using the worst case scenario for heating the test chambers and surroundings is from the coldest ambient temperature (-100C) to the lowest reaction chamber temperature (40C). A 10W heater with 56% efficiency results in 4.05W heating available (includes loss through insulation at worst case). The total time required for this energy change 54.2mins. or 0.9hrs. The power needed assuming a 12V supply is 0.75Amp hrs. 6.3.2.2.0 Cooling The initial cooling problem using the worst case scenario for cooling the test chambers is when the reagent water entering into the test chamber is at the highest temperature allowed (600C) and is needing to be cooled down to the to the highest reaction chamber temperature (370C). These calculations assume the entire system gets to 600C which is probably not true. This assumption is made for simplification and will result in a larger energy requirement that is actually necessary. A 10W heater with 56% efficiency results in 5.6W cooling available (does not include loss through insulation since highest ambient temperature is 300C, loss of energy through the insulation will aid this cooling process. It is not taken into account to provide a worst case scenario). The total time required for this energy change 64.4mins. or 1.07hrs. The power needed assuming a 12V supply is 0.89Amp hrs. 6.3.2.3.0 Thermal Maintenance The maintenance problem for heating and cooling begins with the establishment of an equilibrium temperature. This can be established within the environmental control box; the system may lose or gain 1.55W at a steady rate through the sides of the container. 1.55W will have to be supplied by the heater/cooler continually over the 2 week testing period for a worst case situation. This implies 43.34amp hrs over a two week period. Page 71 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Heating and cooling will be supplied by a TEC acting as a heat pump. It will draw 10W of power at most and supply a max of 5.6W energy for heating or cooling. The insulation will allow for at most 1.55W energy transfer between the inside and outside of the box. The initial heating or cooling for the worst cases can be accomplished in around an hour and for under 1 amp hour of power each. The maintenance of the temperature for 2 weeks can be accomplished for 43.34amp hrs. This is deemed feasible under requirements 4.24, 4.25, 4.26 and 4.27. 6.4.0 Component List A list of the components purchased for the project is shown below in Table 6-4. This list has received most of its components from BioServe in kind. The component list is shown on the next page. Page 72 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 6-4 Component List Component list for MiDAs project AC = Autoclave ST = Sample Transportation RC = Reaction Chamber WC = Water Chamber Component Autoclave Body (2) Autoclave Cap (2) Autoclave Bottom (2) TEC (4) Heat Sink (4) Reaction Chamber (2) Reaction Chamber Cap (2) DC Motor (2) Impeller (2) Mixer Controller (2) Reaction Chamber Environment Peristaltic Pump (2) Pump Mount (2) PharMed Tubing (2) DAQ Embedded CPU Chassis Melamine Insulation (24"x48"x2") Temperature Sensors (6) Press Sensors (4) ISE Package (2) Ultem 1000 (24"x2" rod) 316 Stainless Steel 2 (12"x2.5" rods) Aluminum (48"x48"x0.0625") Bearing Rotary Shaft Motors (2) Syringe Controller (2) Test Tubes (2) Brushless fan Strip Heater (7) 10μm soil Peristaltic Pump Tubes (2) Ball Valve Butterfly Valve (2) O-ring 309 SS for AC prototype Multimeter Wire Control Switchboard Procurement Machined Machined Machined Purchased Purchased Machined Machined Purchased Machined Purchased Machined Purchased Machined Machined Purchased Purchased Machined Purchased Purchased Purchased Purchased Purchased Subsystem AC AC AC AC/RC AC/RC RC RC RC RC RC RC WC WC ST DAQ DAQ Chassis AC/RC AC/RC AC/RC RC RC Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Purchased Screws Purchased AC Chassis RC RC ST DAQ ST Chassis AC/RC ST ST ST ST AC AC Prototyping DAQ AC/RC Chassis Page 73 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 7.0 Project Feasibility and Risk Assessment Author: Elizabeth Newton Page 74 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 7.1.0 Autoclave Prototype The biggest “tall pole” of the MiDAs instrument prior to PDR was autoclaving. The team was uncertain if the chambers could be heated within the power constraints, if the heat losses through the insulation were adequately modeled, and if TECs could heat the autoclave chambers to 121°C. Also, the pressure seal needed to be verified at the elevated temperatures. To mitigate these risks, an autoclave chamber was fabricated out of SS304 stainless steel and tested using TECs, strip heaters, melamine insulation, and temperature and pressure sensors. The setup also allowed us to gain familiarity with the instrumentation, mechanical interfaces between sensors and the chamber, and a rudimentary control system consisting of a LabView code, sensors, and switches. Prior to the fabrication of the autoclave, thermal analysis was done on the chamber using SolidWorks. Figure 7-1 shows the results of that thermal modeling. Figure 7-1 Autoclave Thermal Analysis The model in Figure 7-1 shows a steady-state thermal analysis. A heat source was modeled on the flat, red area and was assumed to reach a temperature of 150°C. The thermal model also assumed a 2W heat loss from every surface, which is comparable to our insulation estimates. This model shows that heating the autoclave from one point creates a very large thermal gradient of nearly 30°C across the chamber. This created a problem because the largest temperature difference a thermoelectric heat pump can achieve is approximately 130°C. That means that if the ambient temperature is 20°C, the TEC would be sufficient to heat the flat area of the chamber to 150°C. However, since the instrument must be able to operate down to -10°C, using only a single-stage TEC to heat will not work. Page 75 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The solution to this problem is using strip heaters. Strip heaters are much more efficient than TECs in heating, and strip heaters can be placed easily under the melamine insulation, and are easier to integrate with fewer components. Strip heaters placed around the exterior of the chamber will ensure much more even heating and a lower thermal gradient throughout the chamber. After performing this thermal modeling, the chamber was constructed. Figure 7-2 shows a SolidWorks model of the prototyped chamber with mounting, lid, and seal interfaces. Sensor Ports Cap Screw Holes O-ring Body Figure 7-2 Autoclave Prototype Model In Figure 7-2, the flat area in front is where the TEC was attached. A silicone O-ring fit into the groove around the top of the body. Temperature and pressure sensors were inserted into the #10-32 threaded sensor ports on the cap. SS304 stainless steel was used for the prototype because SS304 and SS316 stainless steels are very similar, and the aerospace machine shop had some SS304 on hand that we could readily use. Page 76 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 7-3 Autoclave Prototype Setup Figure 7-3 shows the setup of the autoclave prototype. The autoclave itself is encased in the grey melamine insulation on the left. The power supply at the top left provides power to the strip heaters and TEC. The control board and the pressure sensor are shown in the center. The laptop shows the LabView interface that was used to read the temperature and pressure sensors, and to control the solid state relay switches. The pressure sensor was mounted to the cap and read the internal pressure of the autoclave. The temperature sensor was attached to the exterior of the body on the far side of the heater. Figures 7-4 and 7-5, below, show the results of the prototype experiment. 35 pressure (psi) 30 25 20 15 10 5 0 0 20 40 60 80 100 120 140 time (min) Figure 7-4 Autoclave Pressure Results Page 77 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 7-4 shows the results of the pressure readings, as read by LabView. The internal pressure was expected to reach 15 psi (gauge pressure). The actual results showed that the internal pressure was much higher than expected, reaching a maximum of nearly 35 psi. This is because the temperature sensor was mounted outside the autoclave chamber, while the internal pressure is dependent on the highest internal temperature based as tabulated in steam tables. This indicates that the internal temperature was higher than the external sensor readout shows, and thus the internal pressure was higher than was expected. For the final design, the internal temperature can be controlled better to the desired set point of 121°C with minimal overshoot and hence, with lower pressures. This is done by reducing the thermal gradients with strip heaters and placing the temperature sensor in a better location. The pressure dip at around 120 minutes was caused by switching pressure sensors. It was thought that pressure sensor was reading incorrectly when it continued to climb well past the expected point, so a different sensor was inserted in parallel to the recording sensor to validate the sensor reading. Some pressure was released during the addition of the second sensor. Once the temperature reached steady state, the pressure remained constant, but was still higher than expected based on the steam tables due to the thermal gradients between the sensor and the heat source. 140 temperature (deg C) 120 100 80 60 40 20 0 0 20 40 60 80 100 120 140 time (min) Figure 7-5 Autoclave Temperature Results Figure 7-5 shows the temperature readings from the sensor located on the exterior of the autoclave. This graph shows that the exterior temperature on the far side of the heater was able to reach 121°C. This indicates that the heaters are able to heat the autoclave sufficiently, and that the insulation is sufficient to reach the desired target temperature under our power constraints at the tested ambient conditions. It took approximately 130 minutes to heat the exterior of the chamber to 121°C. This time will be extended somewhat when the geological sample and more water are added due to the added heat capacity. Overall, the autoclave prototype was a success, both in performance and experience gained. The strip heaters and TECs will be sufficient to heat the autoclaves to 121°C in a reasonable amount of time. From the thermal analysis, it was decided that placing strip heaters around the outside of the autoclave body will provide more even heating, reduce the gradients and ensure lower pressures with more even temperature distribution at the desired set point. Page 78 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 7.2.0 Mixing Prototype The second tall pole from PDR was mixing. The team was concerned that a small, low-RPM mixer would not be sufficient to mix a 50/50 mixture of small-grain geological sample and water. To mitigate this risk, a mixer was constructed and tested with soil. Originally, there were three mixing options under consideration: ultrasonic, magnetic, and mechanical. Ultrasonic mixing was initially the most promising option due to its heritage in the MECA project. Ultrasonic mixing works through a probe inserted into the mixing chamber. The probe vibrates, sending off pressure waves, and these waves mix the solution. However, the frequency of ultrasonic mixing is dependent on the length of the probe that is inserted into the mixing chamber, with longer probes having a lower frequency. This is a problem because frequencies above 18 kHz create pressure waves strong enough to rupture cell membranes and destroy life. Given the small length of the MiDAs reaction chambers, a probe length small enough to fit in the chamber creates a frequency above 18 kHz. Therefore, ultrasonic mixing was not considered a viable option and was discarded. Our second mixing option was magnetic mixing. Magnetic mixing is common in chemistry applications. A bar magnet is dropped into the mixing chamber and a varying magnetic field below it causes the coupled magnet in the solution to spin. This is an attractive mixing option because there is no complicated interface (rotating shaft seal) between the mixer and the reaction chamber. However, the spinning magnet may interfere with the performance of the electrochemical sensors. This option is still pending research by Tufts University, but until that research is complete, the magnetic mixing option is not being pursued. The final option for mixing was a mechanical coupled mixer, using a dynamic shaft seal. Due to the small size of the reaction chamber, no COTS mixer could be found that met our requirements. Therefore, the MiDAs team designed a mechanical mixer to prototype. The prototype mixer is shown in Figure 7-6. Impeller Chamber Crossbars Gasket Bearing Collar Motor Figure 7-6 Prototype Mixer For the prototype, the impeller and crossbars were made of wood, though in the final design, they will be made of Ultem 1000. The rubber gasket was press-fit over the impeller. The Page 79 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 gasket was then press-fit into the central ring of the bearing collar, which freely rotates within the outer ring. The bearing collar was epoxied into the red chamber lid, which screwed into the chamber. The small metal drive shaft on the motor fit into a hole drilled into the bottom of the wooden impeller. A mixture of approximately 3:1 water and soil was added to the top of the chamber. The soil was 10-micron grain-size soil of unknown chemical composition, obtained from the Geotechnical Department at CU-Boulder. Soon after the mixing prototype was assembled, there was trouble with the way the motor attached to the impeller. This was due to a poor connection between the motor and the wood, which will not be a problem with our actual design. However, this necessitated manual turning of the drive shaft during prototyping. Despite the hand-turning of the drive shaft, the overall mixing design proved successful. The crossbars were able to cause fluid movement around the sides of the chamber. Sediment did accumulate at the bottom of the chamber, however. Therefore, our final impeller design will employ scoop-shaped crossbars very close to the bottom of the chamber to ensure that soil does not accumulate on the bottom. The only concern remaining with the mixing is the bearing collar and shaft seal. Though the collar is designed to be shielded from dirt, it may accumulate sediment, which will cause friction within the bearing. If this is determined to be a problem, there are other interfaces that can be used, such as a sealed gearbox. The floor of the reaction chambers is designed to be replaceable if a new design is needed. 7.3.0 Sample Transportation Prototype Though mixing and autoclaving were the only tall poles identified prior to PDR, sample transportation also proved to be problematic. As more research was done into valves and tubing, it appeared as though transporting the geological sample from the autoclaves into the reaction chamber would be difficult due to friction with the tube walls and obstructed flow through the valve. To mitigate this concern, the MiDAs team performed a prototype experiment of the sample transportation. To perform this prototype, a ¾” ball valve and ¾” internal-diameter tubing were obtained. Some of the 10-micron soil sample obtained from the Geotechnical Department was poured through the valve and tubing to see how much passed through. The soil was weighed before and after being poured through the valve to verify how much soil would be captured in the valve and on the valve and tubing surfaces. Initially, the sample was poured dry onto the closed valve, which was then opened. The sample passed through the ball valve and through a clear tube. Using this method, only about 30% of the sample made it through the valve and tube. This indicates that the soil cannot be satisfactorily transported dry. Page 80 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 For the second test, the dry sample was again poured onto the closed valve, which was opened. This time, however, water was trickled onto the soil to flush it through the tube. Using this method, about 95% of the soil/water mixture passed through the tubing. From these results, it was determined that using water to flush the soil is a satisfactory solution. To mitigate the issue with flow obstruction, the inner diameter of the tubing will be increased to 1” for the final design. This will create a straight, 1”-diameter drop from the autoclave, through the valve and tubing, and into the reaction chamber. There was also some concern about how steam-sterilization would affect the consistency of the sample. It was thought that autoclaving the soil could cause it to bake into a solid brick inside the autoclave, preventing soil transportation. To test this, a sample of 10-micron soil was autoclaved in a standard laboratory autoclave. Several drops of water were added to the soil before being placed into the autoclave. After a full autoclave cycle, the soil had not changed consistency. It was slightly damp, but was still loose and showed no signs of clumping. Based on these tests and the changes to the design of the valve and tubing for the final design, transportation of the soil is less of a concern than it was just after PDR. 7.4.0 Risk Assessment Though prototyping the autoclaving, mixing, and sample transportation mitigated some of the risk associated with the design of the MiDAs instrument, some risk still remains. These risks are shown in Table 7-1. Medium Severity High Table 7-1 Risk Assessment Chart •Autoclave •Mixing •Sample Transport •Machining Time Low •Budget •Reaction Chamber Thermal Control Low Medium High Probability The risks are categorized by low, medium, and high severity and probability. Severity is a measure of how much impact the risk will have on the project and how difficult it is to work around the risk. Probability reflects a risk’s chances of actually coming to pass. Page 81 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Exceeding our budget is currently the lowest severity and probability risk. With our current estimates, we remain $3,500 below the allotted budget of $8,000 (see Chapter 13: Project Management Plan for more details on the budget). The chances of exceeding that budget are small, but if it is exceeded, there is the possibility of obtaining more funding from BioServe, the Undergraduate Research Opportunities Program (UROP), or the Engineering Excellence Fund (EEF). The autoclaving and mixing are also not greatly concerning. Both risks were mitigated somewhat during prototyping. Though the consequences of those risks are high, the chances of them developing into problems are slim. Off-ramps for autoclaving include adding more heaters and insulating the valve interface more if it fails to achieve the necessary temperature. Off-ramps for mixing involve changing the interface from the motor to the reaction chamber through the use of better-sealed bearings and joins. The floor of the reaction chamber and the interface between the valve and the autoclave are removable, which will allow easy redesigns if the designs change and a new interface is needed. It is much easier to fabricate the floor or valve interface than to remake an entire chamber, so time is saved in the case of redesigns. Running over on machining time is a high-probability risk. Due to unforeseen difficulties in the machining process and a general lack of machining experience, it will probably take longer than expected to fabricate parts. This risk is not very severe, however, due to the number of off-ramps. First, some machining will be done over Winter Break to get a head start on the fabrication process. Second, the Lead Fabrication Engineer gained valuable experience in working with stainless steel during the course of the autoclave prototype process. This will allow much faster fabrication of the actual autoclave chamber. Third, extra time for machining has been allotted in the schedule to add a safety factor to the schedule. Fourth, the MiDAs team has access to BioServe’s machine shop. Their fabrication engineer, Don Geering, will be available to assist the team in making parts that are complex or too time-consuming. Despite prototyping, transportation of the sample is still concerning. Problems could develop with the valve interface, such as soil becoming stuck in the valve, preventing it from closing properly. These risks can be mitigated by thoroughly cleaning the valve and its silicone seal before each test and performing a system check prior to testing to ensure that the valves are working properly. Thermal control of the reaction chambers may also be problematic. The TECs should be sufficient to heat and cool the environmental chamber as necessary, but the actual control of the temperature may not work as planned. However, the MiDAs team has the assistance of experts at BioServe who can help improve the design to obtain the specified accuracy and gradients under the power limitations. Overall, there are no major concerns to the MiDAs team at this time. The instrument will be able to be fabricated and tested in the allotted amount of time and money. Though design changes are inevitable, the current design, as laid out in Chapters 8, 9, and 10, is solid and should be very close to the final design. Page 82 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 8.0 Mechanical Design Elements Author: Dave Miller Additional SolidWorks models provided by Sameera Wijesinghe, Jake Freeman (BioServe) and Paul Koenig (BioServe) Page 83 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 This chapter provides details on the individual components used in the system. Mechanical drawings for parts that will be manufactured are found in Appendix A. Figure 8-1 shows a side view of the system with all the insulation removed for clarity. The reaction chamber on the right side has also been removed to show the mixing mechanism within. Sterilization Reagent chambers water chamber TEC with heat sink Tubing Valve Peristaltic pumps Reaction chamber Mixer impeller Mixing motor Mixer couplings Environmental control chamber Figure 8-1 Overall System 8.1.0 Sterilization Chambers The sterilization chambers will sterilize the samples before they enter the reaction chambers. It was determined from the overall system architecture design that two separate sterilization chambers will be required. PDD Requirements: There are two requirements for the reaction samples that drive considerations for the sterilization chambers. The first defines the minimum size of the sterilization chamber while the second helps define the geometry and material needed. 4.13: Reaction Sample Delivery: One pre-measured reaction sample shall be delivered to the test chamber and one pre-measured reaction sample shall be delivered to the control chamber. Both samples shall maintain sterility throughout delivery. o Requirement 4.8: Each reaction chamber shall receive no less than 5 mL and no more than 25 mL of geological sample. Page 84 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.11: Reaction Sample Handling: The reaction samples shall be sterilized in accordance with standard Autoclave techniques. o Standard autoclave techniques implies o 121 C, hold for 15min o Cool to 20 C, hold for 24hrs to allow spores to open o Repeat 3 times There are several requirements that pertain to the heating, cooling, and power available for the sterilization chambers. These requirements are directly related to the thermal analysis performed to model the timeline for the experiment. This analysis is shown after the geometry and materials have been detailed. 4.26: Nominal Power Consumption: Nominal power consumption shall not exceed 30 W. 4.27: Peak Power Consumption: Peak power consumption shall not exceed 30 W for more than 30 seconds. 4.30 Operational Environment: MiDAs shall be able to operate in environments ranging from Antarctica to the Atacama Valley in Chile. o Ambient temperatures ranging from -10 C to 30 C 8.1.1.0 Volume and Dimensions Requirements 4.13 and 4.11 define the minimum internal volume required for each chamber. 5mL water will be added to the chamber for use in autoclaving and 10 mL of space will be provided so the sample is not tightly packed. This implies the total minimum internal volume for the chamber is 40 mL. The internal sterilization chamber geometry will be a cylinder with a tapered base to aid in sample transport after sterilization. The internal diameter of chamber shall be 3.81 cm (1.5 in) to accommodate a 2.54 cm (1.0 in) diameter sample transport pathway leading to the reaction chambers. The sterilization chambers require both heating and cooling to produce the required sterilization temperatures. This directly influences the external sterilization chamber geometry. The chambers shall have four flat surface areas to accommodate the chosen heaters and cooler. Each of these square surfaces shall be 4.06 cm (1.6 in) on a side. Along the upper and lower edges of the chamber, flanges are used for compression fittings for the lid and bottom. Each of these is 0.51 cm (0.2 in) thick. The total height of the chamber is 5.1 cm (2.0in) to accommodate these features. The internal volume of the chamber with these features is approximately 65 mL. This is well above the minimum of 40 mL required, but is the minimum possible with the restraints on the external configuration. The lid to the chambers shall have surface area sufficient to for allow temperature and pressure sensors as well as a pressure release safety valve. It was determined in the overall system architecture that reagent water would be used to flush the samples out of the chambers once the sterilization has been completed. A port for this is also located on the lid. Page 85 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 All of these ports on the lid are threaded for a #10-32 Berwick fitting. The lid is attached to the body of the sterilization chamber by six #4 screws that compress a silicone o-ring to provide sealing. The external diameter of the chamber shall be 6.1 cm (2.4 in) to accommodate these features. The bottom of the chamber is attached to a valve interface using a method similar to the one the lid employs. Six #4 screws are used to provide a compression seal with an o-ring placed on the bottom of the chamber. The housing for the valve selected has a 8.6 cm (3.4 in) diameter. This effectively blocks any access to the bottom of the sterilization chamber from below. Removal of the sterilization chamber from the valve interface is therefore accomplished from above. A flange on the bottom of the chambers is required to provide the necessary area for these screws. This flange is 0.64 cm (0.25 in) thick and has an outer diameter of 8.6 cm (3.4 in). An assembly of the sterilization chamber including the lid, body and valve interface is shown in Figure 8-2. The drawings for these components are in Appendix A. The design of this subassembly which has separate components for the lid, body, and base will facilitate any future changes needed. The lid can easily be redesigned for alternate sensor ports, reagent water supply methods, and potentially automated sample supply methods. The base can also be easily redesigned for interfacing with an alternate valve assembly. By maintaining the same interface between these components and the body, reduced manufacturing and testing times can hopefully be achieved. Temp and Pressure ports #4 screw holes O-ring groove Flange for connection to valve interface 4 flat surfaces: 1 cooler 3 heaters Valve interface 1.0 in sample transport pathway Figure 8-2 Sterilization chamber assembly Page 86 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 8-3 shows a cut-away view of the sterilization chamber body to illustrate the taper at the bottom for sample transport as well as the o-ring groove placement. It also shows how creating flat surfaces on four sides creates thin walls between the heater or cooler surfaces and the inner chamber. The wall thickness between the flat surfaces and the inner chamber is 0.32 cm (0.125in). Standard autoclave techniques require a temperature of 121 C. The pressure required for saturated steam at this temperature is 15 psi above standard. This is an absolute pressure of 29.7 psi or 204774 Pa at sea level on a standard day. For safety concerns, it was desired to know the minimum wall thickness required to contain this pressure. To determine this, thin wall pressure formulas were used. The minimum wall thickness required is 0.03 cm (0.011 in). The chosen wall thickness for the chamber is well above this minimum. Heater or cooler surface Sample pathway O-ring grooves Figure 8-3 Cut-away view of sterilization chamber 8.1.2.0 Material and mass The sterilization chamber, lid, and valve interface will be made of 316 stainless steel to accommodate the high temperatures and moderate pressure required for sterilization as well as to provide for superior corrosion resistance. The total mass of the lid, chamber and valve interface made of this material is approximately 1.2 kg or 2.64 lbs. 8.1.3.0 Thermal analysis The thermal analysis is based on the determination of the internal energy change required to raise or lower a chamber and its contents from a specified starting temperature to an ending temperature. The lowest ambient temperature was set forth in the requirements as -10 C and the highest temperature needed is 121 C for sterilization. The various stages of the sterilization process will each have a different temperature range and calculations for each will be shown in following sections. In general, knowing the required internal energy change, the amount of energy available that can be input into the system and the amount of energy that is leaving the system, it is possible to calculate the time required to complete the change. Page 87 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Internal energy change The internal energy change of a sterilization chamber is U sys U steel U water Q Q [( m)(c p )(T2 T1 )] steel [( m)(c p )(T2 T1 )] water One chamber has a mass of 1.2 kg (2.64 lb) containing 25.0 cm3 (1.5250 in3) of soil (modeled as water here), 5.0 cm3 (0.305 in3) of water (1/2 of which will get vaporized to provide steam for the sterilization). The cp for 316 steel is 452 J/kg K while the cp for water is 4230 J/kg K. In addition to the internal energy change the temperature difference requires, vaporizing half the 5 cm3 of water for steam will require the heater to supply the energy needed for the latent heat of vaporization that the phase change requires. The latent heat of vaporization for water is 2257 J/kg. Stage 1: Heating from worst case ambient temperature to the highest temperature needed for sterilization. This stage requires some of the water added to the soil be vaporized and the energy for this is included in the calculations. The heating requirement considering an upper temperature of 121.0 C and a lower temperature of -10.0 C is an energy change of 115 kJ. Stage 2: Holding at 121 C for 15 minutes. The energy required for this stage will be equal to the energy lost during this time. The loss will be detailed in the insulation section that follows. Stage 3: Cool to 20 C. The water that was vaporized in stage 1 will condense in this stage and the energy lost from the system is included in the calculations. The cooling requirement for an upper temp of 121.0 C and a lower temp of 20.0 C is an energy change of 78 J. Stage 4: Hold for 24 hours at 20 C. The energy required for this stage will be equal to the energy lost during this time. The loss will be detailed in the insulation section that follows. Stage 5: Heat from the lower holding temperature back to the sterilization temperature. The heating requirement for an upper temperature of 121.0 C and a lower temperature of 20.0 C is energy change of 89 kJ. To complete the sterilization phase, stages 2-5 will need to be completed again followed by another holding and cooling time represented by stages 2-3. Energy transfer through insulation A thermal resistance network was used to model the steady state heat loss through the insulation surrounding the chambers. It is assumed that the insulation is in contact with the chamber so there is no convective zone between the chamber and the insulation. It is also assumed that a TEC with a heat sink is attached to one of the flat sides. This area will not be Page 88 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 covered in insulation. The largest heat loss to the surroundings will occur when the there is the greatest temperature difference. It will be less at other times. The convective heat transfer coefficient used for the outer surfaces in this model is h2 25 W m 2C This is only an estimate from other examples using natural convection found elsewhere. This can be increased if forced convection from a fan is used. The thermal resistance network consists of regions of conduction through the insulation, conduction through the TEC, natural convection from the outer surface of the insulation and natural or forced convection from a heat sink attached to the TEC. This heat sink is modeled as having four, 2.54 cm wide, square fins and as being made of an Al alloy. In general, the thermal resistances are found from Rconduction = Thickness kA Rconvection = 1 hAoutside The conduction resistances for the insulation and the cooler can be considered to be in parallel since energy loss will occur through both at the same time. The equivalent resistance is found from Requiv R1 R2 R1 R2 The equivalent conduction resistance and the total convection resistance can be considered to be in series. The equivalent convection resistance is found from adding up the total surface area of the insulation and the heat sink. The total thermal resistance is the sum of the series resistances Rtotal Requiv _ conduction Requiv _ convection The steady state rate of heat transfer is then given by Q Tinside Tsurroundings Rtotal The worst case scenario would be when it is the coldest outside and the hottest inside. The system will lose the most energy under the worst case situation for as long as that condition continues. The model that is used assumes the worst case condition is valid throughout the sterilization process. This will overstate the overall loss in the system, but will provide an upper limit to energy loss. Page 89 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Energy loss through insulation Using the chamber geometry shown above and the thermal resistance networking model for the heat loss at worst case temperatures, the following information regarding the sterilization chamber was found. Melamine insulation can be used. It has a thermal conductivity of 0.03 W/m K and a density of 0.6 lbs/ft3 (9622 g/m3). A 5.08 cm (2 in) thickness of this insulation can surround the sterilization chambers. The steady state heat loss through the insulation and TEC for the worst case temperature differentials of an inside temperature of 121 C and an outside temp of -10 C would be 1.6 W. This insulation will be used throughout the project and the total mass of the insulation was estimated at 383 g (0.844 lbs). Heater/cooler energy The heater and cooler will transfer energy into or out of the system. A thermoelectric cooler (TEC) will provide cooling while strip heaters will provide heating. The TEC considered in the model is assumed to be 56% efficient while the strip heaters are assumed to be 82% efficient per manufacturer’s technical papers. Time and Power required for heating/cooling Using the geometry defined above and the allowed 12W per chamber for heating or cooling, a timeline model for the overall sterilization phase was constructed. The power required is also included here. A 12W heater that is 82% efficient will be able to supply 9.84 W of energy. A cooler that is 56% efficient will be able to supply 6.7 W of energy. The loss of energy through the insulation as shown above will be 1.6 W during the times the worst case temperatures are experienced. A 12W heater/cooler will drawn 1 amps (P=VI) at 12V. At 12V, a 1.6W heater or cooler will draw 0.13 amps. Stage 1: Heating from worst case ambient to highest temperature needed for sterilization. This stage requires some of the water added to the soil to be vaporized and the energy for this is included in the calculations. A 12 W heater with 0.82 efficiency results in 8.2 W heating available (includes loss through insulation). The total time required for one chamber is 231.11 min or 3.85 hours. The power needed assuming a 12V supply is 3.85 Amp hours Stage 2: Holding at 121 C for 15 minutes. The energy required for this stage will be equal to the energy lost during this time and will be at most the energy lost through the insulation (1.6W). Power needed assuming 12V supply to hold for 15 min at high temperature is 0.03 Amp hours. Stage 3: Cool to 20 C. The water that was vaporized in stage 1 will condense in this stage and the energy lost from the system is included in the calculations. A 12 W cooler with 0.56 efficiency results in 6.7 W cooling available (does not include loss). The total time required for one chamber is 193.89 min or 3.23 hours. The power needed assuming a 12V supply is 3.23 Amp hours. Stage 4: Hold for 24 hours at 20 C. The energy required for this stage will be equal to the energy lost during this time and will be at most the energy lost through the insulation (1.6W). The power needed assuming a 12V supply to hold for 24hrs at the low temperature is 3.2 Amp hours. Page 90 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Stage 5: Heating from the lower holding temperature back to the sterilization temperature. A 12 W heater with 0.82 efficiency results in 8.2 W heating available. The total time required for one chamber is 180.8 min or 3.01 hours. The power needed assuming a 12V supply is 3.01 Amp hours. To complete the sterilization phase, stages 2-5 will need to be completed again followed by another holding and cooling time represented by stages 2-3. The actual strip heaters and cooler are detailed elsewhere. 8.2.0 Peristaltic Pumps Two peristaltic pumps will be mounted near the sterilization chambers. These will be purchased parts. They will be connected to the lids of the sterilization chambers through PharMed tubing and a standard #10-32 fitting. These pumps will allow precisely metered amount of reagent water to be added to the sterilization chambers. They will also add 5 mL of water to the sterilization chamber initially for the autoclaving process. Once the sterilization cycles are complete, they will add 25 mL of reagent water to each chamber to flush the sample out of the sterilization chamber. 8.3.0 Reagent Water Chamber A reagent water chamber shall be mounted between the peristaltic pumps. This chamber will be made of Ultem 1000 and have an internal volume of 60 mL. The lid will contain a #10-32 fitting to connect PharMed tubing from the reagent water chamber to the peristaltic pumps. This chamber will also be surrounded by a strip heater to maintain the water in a liquid form. 8.4.0 Valve Assembly The butterfly valve assembly is a purchased product. It provides a 2.54 cm (1 in) sample pathway and when sealed can withstand pressures over 15 psi gauge and temperatures over 121 C. Figure 8-4 shows a SolidWorks model of the value. The opening in the valve is welded to the opening in the valve interface piece shown in Figure 8-2. The valve with the interface piece is then attached to the bottom of the sterilization chambers. During the sterilization process, it was a concern the sample may harden to a point that it would not be able to be transported out of the chamber. The butterfly valve mechanism is one way to aid in the breaking up of the sample before transport. Upon opening, half the valve rotates into the pathway, providing some way to mechanically break up the potentially compacted sample. One area of concern regarding this valve is the initial settling position of the sample in the sterilization chamber. The distance from the bottom of the sterilization chamber to the valve flap will be approximately 1.9 cm (0.75 in). During the experiment, once the sample is added to the sterilization chamber, a portion of it will rest below the inner sterilization chamber on top of this valve flap. The thermal conduction through the valve interface piece may be sufficient to allow the appropriate sterilization temperatures to be reached even at this depth. This will be carefully tested to verify this theory. Page 91 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 8-4 Butterfly valve The valve has a weight of 725g (1.6 lbs). The lower end has tubing attached with a hose clamp to allow sample transport to the reaction chambers which are positioned below this. 8.5.0 Reaction Chambers The two test chambers will receive samples from the sterilization chambers in equal amounts. This sample will be mixed with reagent water in both chambers and a small non-sterile sample shall be added to one chamber. The chambers shall be environmentally controlled for 2 weeks while science data is being taken. It has been decided that the two testing chambers will be housed in one environmentally controlled box. This box shall contain both chambers and all wiring needed for the sensors. PDD Requirements: Requirements pertaining to geometry and mass 4.1 Reaction Chamber Volume: MiDAs shall have two chambers with a minimum internal volume of 50 mL each. 4.4 Reaction Chamber Sensor Capability: Each reaction chamber shall be capable of supporting no fewer than 6 and no more than 18 electrochemical sensors. 4.5 Reaction Chamber Mixing Capability: Each reaction chamber shall have mixing capability such that each geological sample is evenly distributed within the fluid while movement is present at each sensor location. 4.7 Reaction Chamber Material: Each reaction chamber shall be manufactured out of a list of materials provided by BioServe. This list includes, but is not yet limited to, Polysulfone, PharMed, 316 stainless steel, and Ultem 1000. 4.18 Sensor Integration: The electrochemical sensors shall be placed at a minimum height within the reaction chambers to mitigate sample sedimentation effects. This height shall be sufficient to allow the sensors to be fully submerged with a minimum of 5 mL to 10 mL of fluid. Requirements pertaining to thermal control 4.2 Reaction Chamber Temperature: Each reaction chamber shall be controllable within a range of 4°C to 37°C with an accuracy of ±1°C. Page 92 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4.8 Geological Sample Volume: Each reaction chamber shall receive no less than 5 ml and no more than 25 mL of geological sample. 4.16 Reagent Water Delivery: The MiDAs shall aseptically deliver no more than 50 mL (within ± 5% accuracy) of sterile reagent water to each reaction chamber. 4.17 Reagent Water Temperature: The reagent water shall be delivered to the reaction chambers at a temperature not to exceed 60°C. 4.30 Operational Environment: MiDAs shall be able to operate in environments ranging from Antarctica to Atacama Valley in Chile. Ambient temperature ranges from -10 C to 30 C Requirements pertaining to power 4.26 Nominal Power Consumption: Nominal power consumption shall not exceed 30 W. 4.27 Peak Power Consumption: Peak power consumption shall not exceed 30 W for more than 30 seconds. 4.29 Operational Cycle: One operational testing cycle shall be 14 standard Earth days, not including power-up, sterilization, and power-down. 8.5.1.0 Volume and Dimensions Requirement 4.18 states that the lowest row of sensors shall be covered when a minimum of 5 mL of fluid is present. It is assumed that equal portions of fluid and sample are present in the reaction chambers. This implies that 10 mL of volume will need to cover the lowest row of sensors. This drives the chamber body dimensions. Assuming a cylindrical shape for the chamber to minimize sample sedimentation and ease mixing, it is possible with a 2.54 cm (1.0 in) inner diameter chamber to accomplish this by placing the center points of the lowest row of sensors 1.9 cm (0.75 in) above the chamber bottom. There will be 4 rows of 6 sensor ports on the chamber as shown in Figure 8-5. These ports will act as locations for science sensor or as multi-use ports for the addition of the inoculation sample or as a possible means for sample removal. There will also be 4 temperature ports, one along each row. The total length of the reaction chamber body will be 12.7 cm (5.0 in) resulting in a total internal volume of 64 mL. The reaction chambers will have a wall thickness of 0.635cm (0.25 in) to accommodate the sensor ports. The lid will fit over the top of the chamber and seal along an o-ring placed on the outer wall of the chamber body. The top of the lid will contain a section that a 2.54 cm (1.0 in) I.D. hose can be clamped to. This 2.54 cm (1.0 in) pathway is the sample transport pathway. A second view of the lid can be seen in Figure 8-6. Page 93 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The base component of the chamber assembly fits over the bottom of the chamber and seals along an o-ring placed on the outer wall of the chamber. The base will contain a through hole in the center that will contain a sealed bearing for the mixer impeller. The mixer impeller shaft passes through this sealed bearing to a coupling for the mixer motor. The seal between the impeller shaft and the bearing shall be sufficient to hold the sample and reagent water mixture within the reaction chamber for the two week testing period without leaking. The base component also has a flange that contains two #4 screw holes for attachment to the base of the environmental chamber. Figure 8-7 shows the reaction chamber base component. Sample tube attachment Reaction chamber lid O-ring grooves Reaction chamber body Screw holes Muli-use and science sensor ports Temperature ports Reaction chamber base component Figure 8-5 Reaction chamber Figure 8-6 shows a view of the reaction chamber lid from underneath. Page 94 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 8-6 - Reaction chamber lid Figure 8-7 shows the base component of the reaction chamber with the central hole for the sealed bearing visible. Figure 8-7 Reaction chamber bottom The chamber body will be produced by BioServe while the cap and bottom will be produced by the senior projects team. The drawings for these parts are shown in Appendix A. The design of the reaction chamber subassembly which has separate components for the lid, body, and base will facilitate any future changes needed in a similar fashion to the sterilization chambers. The lid can easily be redesigned for alternate sample pathway methods. The base can also be easily redesigned for interfacing with an alternate mixing assembly or changed to a solid cap if magnetic mixing is deemed acceptable. By maintaining the same interface between these components and the body, reduced manufacturing and testing times can hopefully be achieved with this subassembly as well. 8.5.2.0 Material and Mass The reaction chambers, lids, and bases are made of Ultem 1000 because of proven biological and flight tested properties. The total mass of one chamber, lid, and base is 128 g or 0.28 lbs. 8.5.3.0 Thermal Control The thermal control of the chambers shall be achieved through placing both chambers inside one larger environmentally controlled enclosure. This shall be detailed next. Page 95 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 8.6.0 Environmentally Controlled Enclosure The environmentally controlled enclosure will basically be a box that is well insulated and has two TEC attached for thermal control. The box will be made of aluminum to provide thermal conduction pathways to surround the reaction chambers and to give the system some thermal mass to ease temperature maintenance issues. The dimensions for box are a height of 15.7 cm (6.17 in), a depth of 19.4 cm (7.64 in), and a width of 30.5 cm (12.0 in) with a wall thickness of 0.64 cm (0.25 in). It shall have a removable top and side for assembly of the reaction chambers and sample pathways. The base of the environmental chamber shall have two 1.27 cm (0.5 in) holes in it as pathways for the mixer impellers. The mixer motors shall be located below this base. The environmental chamber shall also have an opening sufficient to allow all the sensor and control wiring to pass through to the outside components. 8.6.1.0 Thermal Analysis A method similar to the one used for the sterilization chambers was used to model the thermal conditions for the environmental box. The system modeled was a box made of aluminum that contains both reaction chambers, water as the contents for each chamber and a volume of air surrounding the chambers. The box is insulated on all sides by Melamine insulation, 5.08 cm (2 in) thick. Since the testing period is two weeks, the losses through the walls of this box are the main focus of this thermal analysis. Internal energy change for environmentally controlled box which contains two chambers: U sys U ultem U water U air U Al Q Q 2 *[( m)(c p )(T2 T1 )]ultem 2 * [( m)(c p )(T2 T1 )]water [( m)(c p )(T2 T1 )]air [( m)(c p )(T2 T1 )] Al Initial heating The worst case situation for heating the enclosure is from the coldest ambient temperature to the lowest reaction chamber temperature. The heating requirement considering an upper temperature of 4 C and a lower temperature of -10 C is an energy change of 15.5 kJ. Initial cooling The worst case situation for cooling the enclosure is when the water entering into the test chamber is at the highest temperature (60 C) and needing to be cooled down to the highest reaction chamber temperature (37 C). These calculations assume the entire system gets to 60 C which is probably not true. This assumption is made for simplification and will result in a larger energy requirement than is actually necessary. The cooling requirement considering an upper temperature of 60 C and a lower temperature of 37 C is an energy change of 25 kJ. Page 96 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Maintenance heating/cooling Once an equilibrium temperature can be established within the environmental control box, the system will lose/gain energy through the sides of the box. This is detailed in the next section. Heat transfer through walls of environmentally controlled box: A thermal resistance network will be used once again to model the heat loss through the walls of the box. The system used consists of an aluminum box, the two chambers, their contents and the air surrounding the two chambers. This system will lose or gain energy to the surroundings through the insulation. There will be a convective energy transfer from the air inside the box to the inner surface, a conductive energy transfer through the enclosure walls, a conductive energy transfer through the insulation in the box and finally a convective energy transfer from the outer surface area of the box to the environment. The convective heat transfer coefficients used in this model are: W h1 1 2 for the inside surface area and m C h2 25 W m 2C for the outside surface area. Both these are estimates from the examples found elsewhere. The thermal conductivity used is: W W for the insulation and k 210 for the walls of the enclosure. mC mC The thermal resistance was calculated in a similar fashion to the sterilization chamber model and resulted in a loss of 2.6 W at a steady state worst case situation. The worst case would be when it is the coldest outside (-10 C) and the hottest inside (37 C). The system will lose this much heat under this worst case situation for as long as this condition continues. The thermal modeling done had many sources of error and will be used only as a general guideline at this point. Some of the sources or error include the assumption that the aluminum box, chambers, and contents reach a steady state condition which is probably not true. Additional thermal mass may also need to be added within the environmental chamber to actually provide a stable thermal environment for the reaction chambers for the testing period. The many thermal pathways leading out of the environmental enclosure like wiring, sample tubing, joints between walls and insulation, etc, where not taken into account either. k 0.03 8.6.2.0 Time and Power Required for Heating/Cooling It was calculated elsewhere that during the testing phase, electronics and mixers may require 20W of power. This leaves 10W for the thermal control of the environmental box. Using the geometry defined above with a 10W rated heater/cooler that is capable of supplying 5.6W of energy (only 56% efficient) and insulation allowing only 2.6 W for a worst case energy loss, a power and time model for the overall testing phase was constructed. A 10W heater/cooler Page 97 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 will drawn 0.83 amps (P=VI) at 12V. At 12V, a 2.6 W heater or cooler will draw 0.217 amps. Initial heating The worst case situation for heating the test chambers and surroundings is from the coldest ambient temperature (-10 C) to the lowest reaction chamber temperature (4 C). A 10 W heater with 0.56 efficiency results in 3.0 W heating available (includes loss through insulation at worst case). The total time required for this energy change is 87 min. or 1.45 hours. The power needed assuming a 12V supply is 1.2 Amp hours. Initial cooling The worst case situation for cooling the enclosure is when the reagent water entering into the test chamber is at the highest temperature allowed ( 60 C ) and is needing to be cooled down to the to the highest reaction chamber temperature (37 C ). These calculations assume the entire system gets to 60 C which is probably not true. This assumption is made for simplification and will result in a larger energy requirement than is actually necessary. A 10 W heater with 0.56 efficiency results in 5.6 W cooling available (does not include loss through insulation since highest ambient temperature is 30 C, loss of energy through the insulation will aid this cooling process. It is not taken into account to provide a worst case scenario). The total time required for this energy change 142 min or 2.4 hours. The power needed assuming a 12V supply is 1.96 Amp hours. Maintenance heating/cooling Once an equilibrium temperature can be established within the environmental control box, the system will lose/gain 2.6W at a steady rate through the sides of the container. 2.6 W will have to be supplied by the heater/cooler continually over the 2 week testing period for a worst case situation. 2 weeks is 336 hours. This implies 72.9 amp hours. 8.7.0 Mixer Motors The mixer motors are purchased parts. They will be used to mix the contents of the reaction chambers for the two week testing period. They are located below the environmental enclosure and will be mounted to a base plate. The mixer impeller shaft will protrude below the surface of the environmental enclosure to interface with these motors. There will be a coupling between the motor and the impeller shaft for this interface. The impeller shaft has a T-shaped end which will slide into the coupling and provide for some vertical adjustment capabilities. 8.8.0 External Case The entire structure seen in Figure 8-1 will be enclosed in insulation and will slide into an external case. This can be seen in Figure 8-8 with one of the sides removed and the top partially opened. Page 98 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 8-8 External case This case can be thought of as a PC type case that is made from thin metal sheets. It has the overall dimensions of 46 cm x 46 cm x 39 cm (18 in x 18 in x 15 in). It provides protection for the components inside, is portable and lightweight. The side panels will either slide out or be easily removable with screws for easy user access. Page 99 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 9.0 Electrical Design Elements Author: Charles Vaughan Page 100 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The electrical subsystem consists of a power supply and conditioning, power distribution, sensors, data acquisition, and control system consisting of the heaters, coolers, motors and water pumps. The figure below is a physical representation of the electrical systems distributed throughout the MiDAs system. Electrical Schematic 110VAC Power Source 12V +5V Power Conditioning/ Protection 12V Power Distribution/ Switching CPU Communication (USB) T Dig.Out T P Data Acquisition/ Control Autoclave 1 T AHC2 Temp Control/ Power cut off Autoclave 2 P Water Pump L1 Control Chamber AHC 1,2: Autoclave heater/cooler 1,2 An.In: Analog input An.out: Analog output T=Temperature Sensor P=Pressure Sensor Dig.Out: Digital Output Ambient AHC1 Temp control/ Power Cut off Reagent Water Chamber Control Switch An.In An.out P T P L2 Test Chamber T T Speed control M1 Electrochemical Sensors(12) M2 Speed control IHC: Incubator Heater/Cooler L 1,2: Light 1,2 M1,2: Mixer 1,2 Temp control IHC Reaction Chamber Environment Figure 9-1 Physical Electrical Schematic The diagram illustrates the locations of temperature and pressure sensors as shown in green. The heaters are shown in red, the black items are the motors/pumps, maroon is the power supply, with the white being the data acquisition and control system including the CPU. The torques boxes represent controllers and the light blue boxes represent physical MIDAS systems. This diagram is based on the MiDAs functional layout, and helps to orient the reader in the location and function of the electrical components. Page 101 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 x Computer x 3 6 Input Analog x 0 1 x 0 output x x 1 1 Output x x 4 x 2 1 TEC x 1 TEC AC 2 TEC AC 2 TEC 1 TEC RC Autoclave Autolcave x 4 x 2 x 4 1 Control 2 TEC RC 2 Control 1 Mixer RC RC 2 H2 Autoclave x 2 x 4 x 2 LED's x 2 H1 x 3 x 1 Control 2 Mixer 2 Control Mixer Mixer 2 Autoclave x 2 x 3 x 2 Pump1 x 2 Pump 2 6 Analog x 2 Digital Board Switch 2 2 Sensors 2 Distribution Power Supply Power 9.1.0 Electrical Diagram Figure 9-2 Overall electrical diagram Figure 9-2 is the top-level electrical system diagram, showing subsystem interconnections as electrical buses. The numbers on the buses represents the number of wires present. The power supply is +12VDC and is provided from an external source such as an 110VAC/12VDC power supply, a battery system, or for the Mars instrument, from the lander / rover. Two forms of power will be provided to the MiDAs system regulated +5VDC for the sensors and computer, and +12VDC for all other components. The +5V power will be conditioned to provide voltage stability, because some of the sensors have ratiometric outputs. The 12VDC and 5VDC power will be distributed through the Power Distribution system, which will power the computer, sensors and switch board. All items that receive power directly from the power distribution will be always on (computer, sensors). The power consumption of each of these items will be seen further in the report. The switch board Page 102 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 provides power to all actuators, both on/off and proportional control. The digital outputs will be used to control any of the 9 optically coupled solid state relays. Both of the autoclave heaters will be simple on/off devices. These will simply be used to heat the autoclave chambers up to the desired temperature of 121 degrees C. LEDs will be provided for photosynthetic organisms and will also be on/off controlled. They will be activated when the soil enters the Reaction Chambers. The remaining devices will use commercially off the shelf proportional controllers and will be controlled using the analog outputs. The mixer motors will be powered using linear power amplifiers, powered from 12VDC and controlled by analog outputs, for speed control. The TECs will also use +12V but will be controlled by Pulse Width Modulation (PWM) controllers. 9.2.0 Power Input and Supply +5VDC ? LM340-XX +12VDC 2 T U O N Breaker Circuit ? C Cap .1uF Zener D N D N G G F u 0 0 1 Cap D 1 .22uF ? D ? C Earth Cap D N G 1 ? C V 2 1 + SW-SPST I 3 Amps Supply Power 5 Switch off On U Requirements 12VDC: needs 12VDC (10-15VDC) with up to 3 Amps continuous, 5 Amp. Peak. 5VDC: need 5 VDC (5% accuracy, but .1% stability), <3 Amps Figure 9-3 Power Supply input The power input will receive its power from a DC power supply (110AC/12VDC) or +12VDC from a battery (solar cell, lander, rover, user provided). The voltage level for the 12V power supply is not as important because the items connected don’t have the need for accurate power input. The only specification is that the voltage can not exceed 15V (damage to some components may occur). The Zener diode would protect the downstream circuits by limiting the maximum voltage to 15VDC. The +5V supply does need to be accurate because some of the sensors have ratiometric outputs, proportional to the excitation voltage. The power supply will have a manual on/off switch to start the system. It will also have a 5 Amp circuit breaker protect the system from electrical shorts, which can cause hardware damage and possible fire hazards. The Zener diode will protect against power surges and reverse polarity, limiting the maximum voltage to 15VDC. The capacitor will provide some noise filtering. The +12V will provide power to all the motors and heaters within the system. The sensors will use +5VDC to provide a power source with stable voltage. Because the motors and heaters will be connected to the +12V supply this may cause small voltage fluctuation. This is not a problem for the motors or heaters, but the sensors accuracy relies heavily upon a stable power source because of their ratiometric outputs. Thus by providing a clean +5V Page 103 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 power supply it will help ensure the accuracy of the sensor readings. The Voltage Regulator that creates the +5V can be seen below in figure 9-4. Figure 9.9-4 Voltage Regulator (National Instruments) The voltage regulator has output voltage tolerances of 2% and a line regulation accuracy of 0.01%. This means that the output voltage will be within 2% of 5V (4.9 to 5.1) and the output voltage will stay within 0.01% of the unit-specific output value. The result will be the need to calibrate the ratiometric sensors to the to the actual output voltage. Both C1 and C2 help provide noise filtering to the circuit. Though the output may not be exactly +5V and will vary from regulator to regulator the voltage will be very steady. 9.3.0 Sensor Diagram Requirements Reaction Chamber (PDD doc 4.2) (PDD doc 4.3) Ambient Reaction Sample (PDD doc 4.11) (determined via steam tables) Water Chamber (PDD doc 4.17) Temperature range 4°C to 37°C Temperature accuracy ±1°C Pressure sealed to 1 psi differential to Temperature must reach at least 121°C Temperature range 4°C to 121°C Pressure will reach at least 18 psi Temperature range < 60°C Page 104 of 196 MiDAs December 18th, 2006 ° K 0 K 0 Thermistor 2 ° K 0 Thermistor 3 ° K 0 Thermistor 4 ° K 0 K 0 Thermistor 5 ° t ° t K 0 1 K Thermistor 6 R D N G P R K 0 2 0 D N G N Sig VS + Diagram Sensor D PX139 D N G R 1 2 3 Figure 9-5 Sensor circuit diagram The sensor diagram shows the pressure and temperature sensors. The Pressure Sensors are the Omega PX139 series and the temperature sensors are 10KOhm thermostats. The pressure sensors are self contained items that have built in amplification. The output voltage will be between 0.25 and 4.25VDC. For details on accuracy and minimum resolution within the 12bit A/D system see table 9-1. A picture of the pressure sensor can be seen in figure 9-6. Figure 9-6 PX139 Pressure Sensor (Omega.com) Page 105 of 196 D N G 4 5 6 G AP2 7 8 PX139 9 1 R 1 1 1 1 Sig VS + 1 Q A D 0 K 0 1 PX139 R 1 R 1 t Sig VS + R 1 K 0 1 t D N G AP1 R PX139 1 K 0 1 t R R 1 Sig VS + 1 t Thermistor 1 R P A +5VDC Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 9-1 Sensor system accuracy Sensors Subsystem Data Excitation Output Accuracy Conversion Sensor Specification Pambient Ambient PSIA 5Vdc @2mA 0.25 to 4.25 Vdc +/-0.1% FS 133.33 mV/psia Tambient Ambient °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C PX139-030A4V, Omega, 0 to 30 psia, 0 to 50°C compensated LM34CAZ, National Instruments, -45°C to +148°C Pautoclave1 Autoclave1 PSID 5Vdc @2mA 0.25 to 4.25 Vdc +/-0.1% FS 66.66 mV/psi Tautoclave1 Autoclave1 °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C Pautoclave2 Autoclave2 PSID 5Vdc @2mA 0.25 to 4.25 Vdc +/-0.1% FS 66.66 mV/psi Tautoclave2 Autoclave2 °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C Pchamber Rx chamber box PSID 5Vdc @2mA 0.25 to 4.25 Vdc +/-0.1% FS 400 mV/psi Tchamber1 Rx chamber1 °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C Tchamber2 Rx chamber2 °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C Twater Water chamber °C 5 to 30 Vdc -0.5 to 3 Vdc +/-0.556°C 18 mV/°C PX139-030D4V, Omega, -30 to 30 psid, 0 to 50°C compensated LM34CAZ, National Instruments, -45°C to +148°C PX139-030D4V, Omega, -30 to 30 psid, 0 to 50°C compensated LM34CAZ, National Instruments, -45°C to +148°C PX139-005D4V, Omega, -5 to 5 psid, 0 to 50°C compensated LM34CAZ, National Instruments, -45°C to +148°C LM34CAZ, National Instruments, -45°C to +148°C LM34CAZ, National Instruments, -45°C to +148°C The 10KOhm thermistors change resistance as a function of temperature. In order for the thermistor to function correctly a second 10KOhm resistor with an accuracy of 0.1% will be placed in series before the thermistor. The result will be a temperature dependent voltage drop across the thermistor, which will be measured by the analog input. The thermistors will provide about a 50 mV/degree Celsius signal, and therefore no further amplification is necessary with the 12bit A/D system. In addition, prototype experiments were conducted with the larger LM34 temperature sensor. It provides a linear output voltage of 18mV/C, and covers the entire temperature range of the autoclave. Due to its relative high output voltage, and less stringent accuracy requirements for the autoclave (+/-0.5 C), the LM34 should not require any additional signal conditioning. Page 106 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 9.4.0 Signal Conditioning Currently, only amplified sensors or sensors with high enough output voltage have been selected. However to further reduce the noise in the system, low pass filters (1kOhm, 1.0uF) will be added before the A/D conversion to further reduce noise from the system. Also digital filtering will be used (average of multiple samples) for the analog input signals. This will help eliminate higher frequency radiated ‘white noise’ and even some of the 60Hz line noises easier then with analog filter means. F R Thermistor IHC1 K 0 1 A A IAA ? RBIAS D N CONTROLLER WTC3243 TEC K 0 2 CONTROLLER 8 9 1 7 1 1 1 6 0 1 1 3 4 2 8 9 1 7 1 1 1 6 0 1 2 3 C R L 5 4 3 2 1 ? Control I R I R 2.27MOhm 33.3K D N P R P R ? D N G D 4.9K 4.9K ? C ? Cap 1.5K 1.5K .22uF 1.5K D N G D N G D N ? D N G +5VDC Pump ? Tap Res 2 LED2 LED1 2 H R K 4 5 7 MI 8 MI D N System ? Motor Pump M B 5 2 6 P Control G CLR D N G D N G D N G D N G G + - 6 PI DC PG PG 1 3 +V(ref) 1 H R RWC SCI R -V(ref)1 D N G Peristaltic 1 2 3 4 5 6 7 8 9 +12VDC Computer Figure 9-7 Control Diagram 1 Page 107 of 196 K 5 K 5 K 5 K D N G D N G .22uF 5 Cap C 2.27MOhm G PA75CC 1.5K .22uF B A O N Cap Vs + C IAB + Mixer1 Vs - M B IAA + G 33.3K IAA - 2.27MOhm A A O .22uF D Motor Cap K 1 K 1 C Res3 Res3 2.27MOhm R L 5 4 3 2 1 C F R I R 4 1 D N G WTC3243 TEC K 0 PA75CC 2 B A O G Vs + RBIAS IAB + Mixer1 Vs - M B IAA + - O ° t K 0 1 ° t Control D N G N G Motor IHC2 K 1 K 1 Thermistor Res3 I Res3 R 9.5.0 Control Diagrams CONTROLLER TEC WTC3243 8 9 1 7 1 1 1 6 I P ? 1.5K 1.5K K 5 K D N G 5 D N G .22uF 1.5K K 5 K D N G 5 D N .22uF 1.5K Cap C 2.27MOhm 4.9K R 33.3K R R L 5 4 C 0 1 1 3 4 2 3 2 1 C I P 4.9K R 33.3K R R L 5 4 3 2 1 ? Cap C 2.27MOhm G +5VDC +12VDC Computer 9 8 7 6 5 4 3 2 ? Tap K 3 4 5 6 PI 7 MI 8 MI D N System ? Motor Pump M B 5 2 6 P Control G CLR + - DC PG PG +V(ref) 1 2 SCI Res -V(ref)1 D N G R Pump Peristaltic 1 Figure 9-8 Control Diagram 2 The controllers receive 12VDC switched power from the switch board, and are controlled by analog voltages created by the Diamond-MM32 Digital to Analog Converters. The purely switched (on/off) items include the strip heaters and the LEDs. The strip heaters that will be used are the Kapton HK5544R35.2L12A; it has a resistance of 35.2 Ohms. The result is a power consumption of 4.09 Watts when cold. The LEDS will also run on +12V with a current limiting resistor, and are set to 20mA for 0.24 Watt of power. Page 108 of 196 K 0 Thermistor ° RBIAS K 0 2 CONTROLLER 8 9 1 7 1 1 1 6 0 1 2 3 4 1 WTC3243 TEC K 0 2 RBIAS ° t 1 IHC2 K 0 Thermistor MiDAs December 18th, 2006 t 1 IHC2 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 9.5.1.0 MiDAs December 18th, 2006 Strip Heater Diagram Figure 9-9 Strip Heater (minco.com) The strip heater is a 35.2 Ohm resistance for a theoretical 4.09Watts that will be drawn from a 12VDC supply. Losses will be generated from the internal resistance of the switches and heating. Because 12W of power is necessary to heat the autoclave three heaters will be attached to each autoclave assembled in parallel. 9.5.2.0 Thermostat Figure 9-10 Thermostat (Sensata) To ensure safety on this project a thermostat will be added to each of the autoclaves. The thermostat will have the ability to cut the power to the heater in the event the autoclave becomes too hot. The autoclave will heat to 121C during normal operation. To ensure the safety of the equipment the thermostat will be activated if it reaches 150 C. 176.7C. The thermostat that will be used for this operation is the M1 350040112. This part will have a differential of 22C and a tolerance of 6.7C. The M1 will open on rise, have no mounting brackets and will be platted with Copper. This will give the MiDAs system more then enough protection from heat damage. 9.5.3.0 LEDs Figure 9.9-11 LED (http://www.china-led-manufacturer.com/images/component_ellipse.jpg) Page 109 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The LEDs are only on/off controlled and run at a fixed current of about 20mA, limited by a series resistor. The white LEDs have about 3.5V forward voltage drop at 20mA. With a +12V supply a resistance of 420Ohms is needed. 9.5.4.0 TEC Control The TECs, mixers and Peristaltic pump are initially proportionally controlled, using commercial of the shelf controllers (TEC) or linear power amplifiers (motors). There will be a total of 4 TECs, two on the autoclaves and two in the reaction chamber. All the TECs will have the same design. The TECs will be controlled using the WTC3243 controller from Wavelength Technologies. The schematic provided by Wavelength Electronics can be seen below in figure 9-1210. Figure 9-12 Wavelength TEC control WEC3243 (Wavelength electronics) Upon examining the provided schematic and the electrical diagram above it can clearly be seen that the pictures are different. This is because the BANDGAP VOLTAGE REFERENCE will not be used. The BVR is a built in temperature regulator, based on the resister values with a +5V input the WTC3243 will determine the temperature that the TEC will be controlled too. However because of the desired computer control for bench testing for the MiDAs project, pin 2 will be connected directly to an analog output of the computer Page 110 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 DAQ system. This will allow for temperature regulation of the TEC directly from the computer software instead of the trim pot shown in Figure 9-12. This will provide for more flexibility during initial science testing. A low pass filter will be attached to the analog input at pin 2 to address any high frequency noise on the DAQ line. The signal will have an accuracy of 0.083V/C which will allow for temperature control up to +/-0.5C. This is under the required temperature control of 1 C temperature control. The pin values of the WTC3243 can be seen below: Table 9-2: TEC Pin values 9.5.5.0 TEC (Thermoelectric Cooler) The TEC that will be used for this system will be the CP0.8-127-06. The TEC can operate on up to 16VDC. It can be used for heating or cooling by simply reversing the polarity. A picture of the TEC can be seen below. Figure 9-13 Thermoelectric Cooler (Melcor) Page 111 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Because the TEC can switch its polarity it has the ability to both heat and cool the system, allowing for flexibility on controlling temperatures of the reaction chamber both above and below the ambient temperature. Combined with the proportional controllers from Wavelength (WTC3143) will allow for very tight temperature control of the Reaction chamber. The specifications for this TEC can be seen in the table below. Table 9-3: TEC specifications (Melcor) 9.5.6.0 Fan Because of the large amount of heat that will be generated by the operation of the TECs and their controllers fans will be needed. In a space flight application the cooling of the TEC and its control would be the responsibility of the rover thermal control system the fan that will be used is the Melcor BP401012H. The selected fan will provide enough volumetric flow rate to properly cool the system even at altitudes of 5000m (5.4*10^4Pa). The fan operates off of 12V at .13A. The addition of this fan to the TEC control will add an additional 1.56W of power consumption. 9.5.7.0 Peristaltic Pump The water pump that was chosen for this project was the P625 Peristaltic Pump from Instech. This pump has been used previously for spaceflight applications. There will be two pumps operating in the system, one for each autoclave chamber. A single pump design with a pinch valve was also explored and still may be used because of the high cost of the pump, The P625 was chosen because it allows accurate fluid metering, and the motor speed control with feedback is built into the pump assembly. The schematic for the pump controller on the pump body can be seen below: Page 112 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 9-14 Peristaltic pump controller connection schematic (Instech) The peristaltic pump will operate off of +12V and will normally use a trim pot to control the motor speed and therefore the water flow. This means that the water flow can be adjusted by changing the resistance on the trim pot, and therefore the control voltage to the speed controller (pin2). There is no need for active control over the water flow from the computer; therefore this manual control for calibration purposes is acceptable. The water flow is controlled by time operation of the motor at fixed and known flow rates. The trim pot will be adjusted during assembly to ensure the desired calibrated water flow. 9.6.0 Mixer Control Requirements (PDD 4.1.1) Each reaction chamber shall have mixing capability such that each geological sample is evenly distributed within the fluid while movement is present at each sensor location. (PDD 4.1.2) Verification will be through inspection. The mixers will be initially speed controlled by the computer for development. A later design after testing may use fixed motor speed. The speed is controlled by a linear power Page 113 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 operational amplifier (op. amp.), using an analog voltage from the computer to the power op. amp. input the device that was chosen for the motor control was the APEX PA75CC, which is a dual power op amp. This will require additional external connections to complete the motor control device. The schematics of the PA75CC can be seen in the figures below: Figure 9-15 Controller layout (from APEX) Figure 9-16 APEX PA75CC Figure 9.15 shows the electrical schematic of the control system. Figure 9.16 shows the connectivity to the pins of the PA75CC. The Rs resisters seen in figure 9.15 are used to control the current flow, and to ensure the chip does not overload. Because the currents required for the motor used is small (100mA), these current-resisters were not used and can be considered 0 Ohm (No current limit). The values of Rf and RI will determine the amplifier gain. Rf has a value of 20Ohm and RI has a value of 10Ohm, which translates into a gain of 2x. For a gain of this size an input voltage from the analog input will allow for a motor Page 114 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 voltage of 0-10VDC from a DAQ output of 0-5VDC. The motor specifications can be seen below. 9.6.1.0 Mixer The mixer motor that will be used is the Faulhaber 1224. The motor will be run using +12VDC and will draw approximately 1.95W of power. With at +12VDC input the speed constant is found to be 1151rpm/V. The torque constant for this motor is 8.3 mNm/A. Further testing will be done to find the speed and therefore the required torque that will best mix the soil solution. The table below gives a further description of the motor selected for the mixing. Table 9-4 Mixer motor specifications (http://www.micromo.com/uploadpk/1224_SR.pdf) Page 115 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 9.6.2.0 MiDAs December 18th, 2006 Computer Figure 9-17 On board computer, an embedded PC104 controller from Kontron (MOPSlcd6LX) The computer that will be used in this project will be the MOPSlcdLX. This is a micro computer that will be onboard the experiment. It has an AMD LX800 processor, which operates at 500MHz. It does not require a fan to operate and can be operational in environments between 0 and 60 degrees C. The MOPSlcdLX operates from only a single 5VDC supply, with less then 200mA current draw (0.9 Watt for CPU without periphery connected). For external access it is equipped with two USB ports and an Ethernet port. This computer will have more then enough capacity to provide for the experiment. A solid state compact flash disk (4GB, power consumption not included in above CPU power draw) with Windows and LabView will be used initially (flight software would be in C++ and Linux operating system). The CPU (MOPSlcdLX) will have a Diamond systems MM32 data acquisition and control board connected on the PC104 expansion bus. Its power draw (410mA at 5VDC typ.) is also not included in the 200mA/5V supply to the CPU. 9.7.0 DAQ Requirements (PDD 4.19) Sensor Data Collection Rate (PDD 4.19.1) The electrochemical sensors shall have a data collection rate of 1 measurement per minute per sensor. (PDD 4.1.2) Verification will be through demonstration. Page 116 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 (PDD 4.20) Sensor Data Acquisition (PDD 4.20.1) All data taken through the sensors shall be collected and stored for analysis. (PDD 4.20.2) Verification will be through analysis and demonstration. (PDD 4.20) Sensor Data Accessibility (PDD 4.21.1) The scientific and engineering status data shall be accessible to users throughout the experiment. (PDD 4.21.1) Verification will be through demonstration. Figure 9-18 Data Acquisition (Diamond MM-32 from Diamond systems) The DMM-32X-AT is one of the most advanced data acquisition devices Diamond system sells. Because of the number of inputs needed for the project two will be used. The DiamondMM32 has 16 bit resolution with a maximum sampling rate of 250 KHz. The circuit has built in low drift circuitry which will ensure a very accurate voltage reference. The design of the chip also took into consideration noise problems with digital and analog signals. The chip was designed to have digital and analog regions so as to minimize noise. Any wires from one region that cross into the other are heavily shielded. Each DAQ is equipped with 32 analog Page 117 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 inputs. The analog inputs are where the sensors will read in their voltages. These inputs are sent into a built in amplifier, which will reduce the error found in the system. There are 4 analog outputs per circuit, but because 2 DAQs will be used a total of 8 will be available for control. Finally, 24 digital outputs are available per DAQ. The digital outputs will be used to control the switch board. Further description of the DAQ can be seen in the table below. Table 9-5 DAQ system Page 118 of 196 D N V 2 1 + V 5 6 3 5 3 D N G + 0 G 8 3 7 3 4 9 V 5 + D N G 4 3 Distribution Power V 5 + 2 3 V 2 1 + 0 3 D N G 8 2 V 2 1 + 6 2 D N G 4 2 V 2 1 + 2 2 D N G 0 2 V 2 1 + 8 1 7 1 Sig D N G 6 1 5 1 Sig V 2 1 + 4 1 3 1 Sig D N G 2 1 1 1 Sig V 2 1 + 0 1 9 Sig D N G 8 7 Sig V 2 1 + 6 5 Sig D N G 4 3 Sig V 2 1 + 2 1 Sig Items Switched Board Switch Output Digital Diagram Wire System D N G D N G V 2 1 + V 2 1 + Computer Supply Power Sig D N G Sig V 5 + D N G AT2 V 2 1 + Sig Pump D N G D N G V 5 + V 2 1 + AT1 Heater2 Sig D N G D N G V 2 1 + V 5 + Heater1 Sig D N G D N V 2 1 + V 5 Pump T Sig Sig C + V 5 + T G D N G A Sig Sig D N G D N G Sig V 2 1 + Sig V 5 + D N G D N G Sig V 2 1 + D N G V 5 + D N G V 2 1 + V 5 + TEC4 T T D N G Sig Sig V 2 1 + Sig D N G D N G Sig D N G V 5 + V 2 1 + D N G V 5 + Sig V 2 1 + D N G TEC3 T R Sig V 5 + V 5 + D N G Sig Sig D N G V 2 1 + D N G D N G V 2 1 + Sig V 5 + V 5 + D N G Sig TEC2 P R V 2 1 + D N G V 5 + V 5 + Sig V 5 + D N G D N G D N G Sig V 2 1 + V 2 1 + V 5 + D N G V 5 + V 5 + TEC1 AP2 D N G Sig Sig V 2 1 + Sig D N G D N G Sig D N G V 5 + V 2 1 + D N G V 5 + Sig V 2 1 + D N G Mixer2 AP1 Sig V 5 + Sig D N G Sig Input DAQ D N G V 2 1 + D N G V 2 1 + V 5 + inputs Control Mixer1 P A 9.7.1.0 3 V 2 1 + Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Wire Diagram Figure 9-19 Wire Diagram The wire diagram was created to understand how many connections are going to be needed for the switch board and the computer interface. The switchboard can be seen in figure 9.19 below. The optically coupled MOSFET solid state relays (NAIS ACQ202, 2 Amps each) are not shown on this wire diagram, but will be shown on a separate circuit board design drawing. Figure 9-20 Switch board Page 119 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Currently 6 Analog output signals are needed to run the motors and TECs, 10 analog inputs are needed for the sensors, and 11 digital outputs are needed to run the switch board. When the voltages and grounds are added to the system a total of 42 connections are needed in the switch board. The power distribution will need a total of 25 connections to power the sensors, computer and switch board. The Drawing Tree summarizes all the parts necessary to construct the electrical system. Table 9-6 Drawing Tree Subsystem/Item Drawing Number Drawing Name Drawn by Electrical Block Diagram MiDAs-OS-A-200-01 Power Subsystem Power Supply Power Distribution MiDAs-OS-A-210-01 MiDAs-OS-P-211-01 MiDAs-OS-P-212-01 Power System CV Sensor Subsystem Wire Harness Pressure Sensors Temperature Sensors MiDAs-OC-A-220-01 MiDAs-SC-P-221-01 MiDAs-SC-P-222-01 MiDAs-SC-P-223-01 Sensor Schematics CV Control Schematics CV Control Subsystem Mixer/Control 1 Mixer/Control 2 Autoclave TEC/Control 1 Autoclave TEC/Control 2 RC TEC/Control 1 RC TEC/Control 2 Wire Harness Fan Switch Board Strip heaters LEDs Wire Harness Water Pump Data Acquisition and Control Computer Analog input Digital input Analog Output MiDAs-OS-A-230-01 CV MiDAs-SC-P-231-01 MiDAs-SC-P-232-01 MiDAs-SC-P-233-01 MiDAs-SC-P-234-01 MiDAs-SC-P-235-01 MiDAs-SC-P-236-01 MiDAs-SC-P-237-01 MiDAs-SC-P-238-01 MidAs-OS-A-240-01 Switch Board Layout CV Computer Interface CV MiDAs-SC-P-241-01 MiDAs-SC-P-242-01 MiDAs-SC-P-253-01 MiDAs-SC-P-254-01 MidAs-OS-A-250-01 MiDAs-SC-P-251-01 MiDAs-SC-P-252-01 MiDAs-SC-P-253-01 MiDAs-SC-P-254-01 Page 120 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 9.8.0 Powered Items The following is a list of elements of the Midas project that will require power. The constant power is the summation of the systems that will always be running. The Switched Power is the systems that can be turned on and off, and the Controlled power systems are those that need to have power control similar to a light switch dimmer. Constant Power Computer CF disk, 4GB with IDE controller DAQ Autoclave temp sensor Autoclave Pressure sensor Reagent Water Temperature sensor Test chamber temperature sensor Reaction chamber Pressure Sensor Control chamber temperature sensor Ambient Temperature Sensor Ambient Pressure Sensor Total Constant power draw Number Table 9-7 MiDAs Powered Items Voltage(V) Amps(A) Power(W) 1 1 5 5 0.200 .02 1.00 2 2 5 5 0.410 x 2 0.0005 4.10 (2.05 ea) 0.0025 2 5 0.002 0.01 1 5 0.0005 0.0025 1 5 0.0005 0.0025 5 0.002 0.01 1 5 0.0005 .0025 1 5 0.0005 .0025 5 0.002 .01 5 1.0485 5.24 1 1 Switched Power Water Pump Strip heaters LEDs 2 7 2 12 12 12 0.125 0.34 0.02 1.5 4.09 0.24 Controlled Power Mixers Incubator TEC Autoclave TEC 2 2 2 12 12 12 .1625 1 1 1.95 12 12 Page 121 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 9-8 Electronics Parts List Electronics Parts List # used Power System On/off switch Circuit 5Amp Breaker Capacitor .22uF Diode Capacitor .22uF Capacitor .1uf Voltage Regulator 18 gage wire (Blue) Sensors Pressure Sensor Temperature Sensor Resistor 10kOhm 26 gage wire (Blue) 24 gage wire (green) Control Motor Control Company part # 1 1 1 1 1 1 1 Philmore SW-SPST American Electrical Inc C5A1P AVX Corporation 08053C224JAT2A Diodes Inc. SMAJ15A-13-F AVX Corporation 08053C224JAT2A AVX Corporation 06033C104JAT2A National Semiconductor LM340-XX 4 6 6 Omega PX139 2 APEX PA75 Mixer 10Ohm Resistor 20Ohm Resistor TEC control 2 2 2 2 Faulhaber 1224 TEC 4.99KOhm Resistor 50W 33.3KOhm Resistor 10W 1.5KOhm Resistor 5W 5Ohm Trim pot 10W Switch Peristaltic Pump 10KOhm Trimpot 10W 2 4 4 8 8 4 2 2 Melcor CP0.8-127-06 Strip heater LED 7 2 Kapton HK5544R35.2L12A Thermostat 7 Fan 18 gage wire (Blue) 18 gage wire (Red) 26 gage wire (Green) Computer CPU DAQ 18 gage wire (red, back) 26 gage wire (white, black) Flash Drive 4 Klixon M1 350040112 Melcor BP401012H 1 2 Kontron MOPSlcd6LX Dimond MM-32 1 GE Sensing YM120D370N100 Honeywell SC5E10 Honeywell SC5E10 Honeywell SC5E20 Wavelength WTC3243 Honeywell CMC5010 Honeywell VC10F33 Honeywell SC5E1.5 Honeywell VP10FA5 Philmore SW-SPST Instech P625 Honeywell VP10FA5 Bourns Inc. 51AAD-B28-D15L Transcend 4GB Page 122 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 10.0 Software Design Elements Author: Steven To Page 123 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Requirements (PDD 4.2) Reaction Chamber Temperature (PDD 4.2.1) Each reaction chamber shall be controllable within a range of 4°C to 37°C with an accuracy of ±1°C. (PDD 4.3) Reaction Chamber Pressure (PDD 4.3.1) Each reaction chamber shall be sealed to a pressure of 1 psi differential. (PDD 4.5) Reaction Chamber Mixing Capability (PDD 4.5.1) Each reaction chamber shall have mixing capability such that each geological sample is evenly distributed within the fluid while movement is present at each sensor location. (PDD 4.11) Reaction Sample Handling (PDD 4.11.1) The reaction samples shall be sterilized in accordance with standard Autoclave techniques. (PDD 4.16) Reagent Water Delivery (PDD 4.16.1) The MiDAs shall aseptically deliver no more than 50 mL (within ±5% accuracy) of sterile reagent water to each reaction chamber. (PDD 4.17) Reagent Water Temperature (PDD 4.17.1) The reagent water shall be delivered to the reaction chambers at a temperature not to exceed 60°C. (PDD 4.19) Sensor Data Collection Rate (PDD 4.19.1) The electrochemical sensors shall have a data collection rate of 1 measurement per minute per sensor. (PDD 4.20) Sensor Data Acquisition (PDD 4.20.1) All data taken through the sensors shall be collected and stored for analysis. (PDD 4.21) Sensor Data Accessibility (PDD 4.21.1) The scientific and engineering status data shall be accessible to users throughout the experiment. (PDD 4.22) MiDAs Status Warnings (PDD 4.22.1) MiDAs shall provide caution, warning, and instrument status to external ground support equipment. (PDD 4.23) MiDAs command (PDD 4.23.1) MiDAs shall received commands from external ground support equipment. Software will be used in several manners. First, the software will be use for data acquisition. This is to verify sections 4.2, 4.3, 4.11 and 4.17 from the PDD document as well as satisfying requirements 4.19, 4.20 and 4.21. Second, the software will also have to control all the necessary components, such as heaters, coolers and motors to satisfy all requirements listed above. To achieve these requirements, National Instrument’s LabView was chosen as the programming language for its ease of use and functionality. To run the software program, LabView will be installed onto the PC104 embedded CPU (Kontron MOPSlcdLX). The Page 124 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Kontron MOPSlcdLX will allow for user access via Ethernet connection or by plugging in a keyboard and monitor to its respected ports. Once installed onto the PC104, the software will require a Data Acquisition Card in order to read the necessary data. Diamond’s MM32 DAQ card will used for its available number of sensor inputs allowing us to collect data from all sensors as well as for its number of outputs, to allow for control of our systems. Control of our system will be achieved in two ways. First is simple on/off control in which power is turned on or off to the desired component. This is accomplished via the DAQs digital I/O outputs toggling various switches. Second is proportion control in which power is proportionally adjust to control the speed or temperature of the desired component. This is accomplished via the DAQs analogue outputs coupled with an APEX PA75CC controller. The controller is ideal for the Autoclave temperature control in which various temperatures will need to be held at different times. In this case, the DAQs analogue outputs send a desired voltage to the controller which then interprets this voltage as the desired temperature to hold to. A simple description of the execution of the software is shown in figure 10-1. Figure 10-1 Simplified execution The given times along the time line are approximate values based on initial calculations of the autoclave thermal properties. Circles indicate user inputs and outputs. A more detailed description follows in Table 10-1 which outlines all the necessary controls for one testing system. Page 125 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Table 10-1: One cycle control This table imposes some small safety margins to ensure proper temperatures have been reached. For example, the autoclave heating temperature is 121°C, but the control specifies 125°C to make sure. Page 126 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The sensor diagram to run the experiment is described below in Figure 10-2 and 10-3 to show the inputs and outputs during heating control of the autoclave and the reaction chamber. 4| Power (W) Proportional control 4| CTemp (V) 5| On/off Power (W) 4| Temp (V) Heater 6| Qin (W) On/off control 1| Temp (V) 3| High/Low Autoclave DAQ 1| Press (V) 2| Yes/no/ CTemp (bin) 2| Temp (bin) Software control Is it heating? Is it cooling ? Is it holding constant temp? 5| Qout (W) Cooler 2| Temp (°C) User 2| Press (psi) 4| Power (W) Figure 10-2 Autoclave Sensor Diagram Step 1 The DAQ inputs the sensor data (in the form of analogue volts). Internally, the DAQ converts the analogue voltage into a digital bit. Step 2 The DAQ then sends the digital values to the embedded CPU which runs the software and determines whether to turn the heater or cooler on or off and at what temperature the proportional control (CTemp) should hold the autoclave to. The software must also be capable of digitally filtering noise, and converting the digital inputs into a corresponding measurement (psi, °C, etc) for user to monitor if desired. Step 3 The DAQ again converts the digital input from the CPU to a digital High/Low signal for the on/off control Step 4 The On/off control determines relays power to either the proportional control for the heater or to the cooler. The DAQ, when applicable, converts the digital input form the CPU to an analogue volt output for the proportional control. Step 5 If the heater is on, the proportional control sends power to the heater and continues to monitor the Autoclave to maintain the correct temperature. If the cooler is on, it begins to cool the Autoclave by removing heat. Step 6 The Heater heats the Autoclave as per the proportional controller’s instructions. Page 127 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 4| Heating/Cooling Proportional control 4| CTemp (V) 5| On/off/ Temp (V) 4| Temp (V) TEC 6| Qin/Qout (W) Heat/Cool control 1| Temp (V) 3| High/Low DAQ Reaction Chamber 1| Press (V) 2| Yes/no/ CTemp (bin) 2| Temp (bin) Software control Is it heating? Is it cooling? Is it holding constant temp? 2| Temp (°C) User 2| Press (psi) Figure 10-3 Reaction Chamber Sensor Diagram The reaction chamber diagram is similar to the autoclave sensor diagram except for the type of input received from the TEC. Since the reaction chamber will be controlled solely by the TEC the Heat/Cool control must be able to reverse the polarity of the power being provided to the TEC which can be accomplished with four switches. Figure 10-4 below is the LabView vi program for the autoclave temperature control. This serves as an example of how the sensor diagram, Figure 10-2 is implemented via the procedure of Table 10-1. Page 128 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 10-4 LabView vi Autoclave Temperature Control While Figure 10-4 may appear complicated, most of the components are simply visual overhead for the user to see what software is doing. Broken down to just the major functions it becomes a much simpler program Figure 10-5 below. Figure 10-5 Software Function Tree Page 129 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 11.0 Integration Plan Author: Shayla Stewart Page 130 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 11.1.0 Instrument Assembly Figure 11-1 shows the assembly flow diagram for the MiDAs instrument. The gray components indicate the purchased or donated parts, while the white indicates that the component will have to made or manufactured by the MiDAs team. The assembly starts from the bottom and moves upward to the integration with the chassis, which is made up of an outer case and an upper shelf inside. Figure 11-1 Assembly Flow Diagram Not every subsystem has to be put into the chassis individually. Figure 11-2 shows the larger assemblies that will be inserted into the overall chassis. The section marked ‘1’ consists of the mixer impeller, reaction chamber bottom, body, and cap as well as the PharMed tubing and autoclave bottom-valve interface used for the sample transport from the autoclave to the reaction chambers. Each of these components is sterilized in an autoclave provided by BioServe to kill any living organisms present on each piece. This section is then assembled outside of the instrument in a clean and sterile environment. This ensures that no contamination or introduction of living organisms will occur during the assembly process. This entire section is then slid into the chassis from the side through cutouts in the chassis shelves and the impeller will be fitted into the motor bearing. This section is a completely Page 131 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 closed and sterile environment for the experiment, which is then screwed in. The section labeled ‘2’ shows the overall sterilization assembly including the autoclave cap, body, and the TEC assembly with the heat sink. This is then screwed onto a shelf attached to the bottom of the chassis. 2 1 Figure 11-2 Overall Assembly 11.2.0 Autoclave Functional Test Plan PDD requirement 4.11 states that the reaction samples must be sterilized through standard autoclaving techniques. This requires that the autoclave chambers be heated to 121°C and held for 15 minutes before being cooled back down to 20°C and holding for 24 hours. This cycle must be repeated three times because of the possibility of spores in the sample. Cooling the sample to close to room temperature and holding allows for the spores to diminish their protective shells so that they will be killed on the next autoclave cycle. The sample must be transported from the autoclaves to the reaction chambers once the sterilization is complete. To ensure that there is enough of the sample to get a good test and control, at least 90% of the sample must reach the reaction chambers when the reagent water is pumped through. Figure 11-3 shows the functional test plan for the autoclaves. The thermal control on the autoclaves consists of three strip heaters wired in series and a thermoelectric cooler (TEC). The sample transport from the autoclave to the reaction chambers involves a butterfly valve at the bottom of the autoclave and is also a function of the sample consistency. If the soil is too dense or sticky, it may be difficult to move it in the chamber. The thermal control and sample transport will need to be tested prior to integrating the autoclave into the entire system. The details of the testing will be outlined in section 12 of this report. Page 132 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 TEC Thermal Control Autoclave Sample Transport Strip Heaters Butterfly Valve Heat from -10°C to 121°C Hold for 15 min Cool to 20°C Repeat 3 times Transport 90% of sample when reagent water pumped through Sample Consistency Figure 11-3 Autoclave Functional Test Plan 11.3.0 Reaction Chambers PDD Requirement 4.2 states that each reaction chamber must be controllable within a temperature range of 4-37°C with an accuracy of ±1°C. Aside from the chamber thermal control, there is a mixing requirement as stated in PDD requirement 4.5. The sample/water solution in the chambers must be mixed so as to distribute the sample and minimize sample sedimentation at the bottom and sides of the chamber. There also must be fluid movement at each of the sensor on the side so that accurate readings are possible. Figure 11-4 shows the functional test plan for the reaction chambers. The thermal control for the reaction chambers is performed within the reaction chamber environment with two TECs placed near the reaction chambers. The mixing within the chambers involves a small motor and the designed impeller. The thermal control and mixing capability will need to be tested prior to integrating the reaction chambers into the entire system. The details of the testing will be outlined in section 12 of this report. Reaction Chamber Thermal Control TEC Motor Mixing Impeller Maintain temperature between 4°C and 37°C Maintain fluid movement around sides; Maintain minimal sedimentation on sides and bottom of chamber Figure 11-4 Reaction Chamber Functional Test Plan 11.4.0 Data Acquisition and Control PDD requirement 4.20 states that all of the data taken through the sensors shall be collected and stored for analysis. The instrument must also be able to receive commands and provide caution, warning, and instrument status as specified in PDD requirements 4.22 and 4.23. Page 133 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Figure 11-5 shows the functional test plan for the Data Acquisition and Control subsystem. The collection, storage, and command capabilities are dependent on the hardware as well as the interface and LabView software. These capabilities must be tested prior to its overall integration into the system. The details of the testing will be outlined in section 12 of this report. DAQ & Control Collection & Storage Command Interface Software Collect & store data from each sensor Receive commands from SW Provide caution, warning, status signals Figure 11-5 DAQ Functional Test Plan Page 134 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 12.0 Verification and Test Plan Author: Shayla Stewart Page 135 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 12.1.0 Design Requirement Verification Plan Table 12-1 shows the verification plan for each requirement stated in the PDD and in Table 1-2. The verification methods are defined as analysis (A), test (T), inspection (I), and demonstration (D). Table 12-1 Design Requirement Verification Plan Verification Requirement Method Reqt. # Title PDD 4.1 Reaction Chamber Volume ≥ 50 mL PDD 4.2 Reaction Chamber Temperature 4 – 37°C ±1° A, T 1 psi differential A, T 6 – 18 sensors A, I Analysis and design of the chamber geometry and by visual means Fluid movement, minimal sediment A, I Analysis of the flow pattern generated during mixing and by visual/video means. ≥ 4 multi-use ports A, I Analysis and design of the chamber geometry and by visual means Polysulfone, PharMed, 316 SS, Ultem 1000 A Structural and thermal analysis of the reaction chambers. 5 – 25 mL I, D Simple volume measurement prior to insertion into autoclave ≤ 1 mL I, D Aseptic delivery A, T PDD 4.3 PDD 4.4 PDD 4.5 PDD 4.6 PDD 4.7 PDD 4.8 PDD 4.9 PDD 4.10 Reaction Chamber Pressure Reaction Chamber Sensor Capability Reaction Chamber Mixing Capability Reaction Chamber MultiUse Port Reaction Chamber Material Geological Sample Volume Inoculation Sample Volume Inoculation Sample Reception I, D Verification Simple volume measurement. Thermal analysis of the environmental chamber geometry; Test by means of temperature sensors within the reaction chamber environment Pressure decay of sealed chamber by means of pressure sensors within the reaction chamber environment Simple volume measurement prior to insertion into reaction chamber Analysis of sample transport; Sterile swabbing of wetted surfaces with biological culture test Thermal analysis of the autoclave chambers; Testing by means of temperature/pressure sensors through cap and autoclave indicator tape PDD 4.11 Reaction Sample Handling Steam autoclave sterilization A, T PDD 4.12 Inoculation Sample Handling No sterilization A, D Thermal analysis making sure sample does not get near autoclaves; Demonstration PDD 4.13 Reaction Sample Delivery Aseptic delivery, equal volume to each chamber A, T Analysis and testing of sample transport through valve; Sterile swabbing of wetted surfaces with biological culture test Page 136 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Analysis of sample transport; Sterile swabbing of wetted surfaces with biological culture test Thermal analysis of reagent water chamber; Test by means of temperature sensors within chamber Analysis of peristaltic pump with volume and flow control; Simple volume measurement after flow through pump PDD 4.14 Inoculation Sample Sterility Aseptic delivery A, T PDD 4.15 Reagent Water Containment Contained as solid & liquid A, T PDD 4.16 Reagent Water Delivery ≤ 50 mL ±5%, aseptic delivery A, I PDD 4.17 Reagent Water Temperature < 60°C A, T Thermal analysis; Test by means of temperature sensors within chambers PDD 4.18 Sensor Integration A, I Analysis of reaction chamber geometry; Simple volume measurement PDD 4.19 Sensor Data Collection Rate A, T Analysis and testing of the DAQ/command software PDD 4.20 Sensor Data Acquisition A, T Analysis and testing of the DAQ/command software. PDD 4.21 Sensor Data Accessibility PDD 4.22 MiDAs Status Warnings PDD 4.23 MiDAs Command PDD 4.24 Field Power PDD 4.25 Laboratory Power PDD 4.26 PDD 4.27 Nominal Power Consumption Peak Power Consumption Bottom row submerged in 5 – 10 mL 1 measurement per min per sensor All data collected & stored Accessible during experiment Provide caution, warning, status signals Receive commands Operate on 10 – 30W @ 12VDC in field Operate on 10 – 30W @ 12VDC in lab ≤ 30W @ 12VDC > 30W for ≤ 30 sec Taken apart, sterilized, reassembled D A, T A, T Demonstration of data transfer from embedded CPU Analysis and testing of the DAQ/command software with set max temperature and shutoff abilities Analysis and testing of the command software A, T Analysis of the power consumption; Test using multimeter within circuit A, T Analysis of power consumption; Test using multimeter within circuit A, T A, T PDD 4.28 Unit Disassembly A, D PDD 4.29 Operational Cycle 14 standard Earth days A, T PDD 4.30 Operational Environment Antarctica to Atacama Valley (-10 to 40°C) A, T Analysis of power consumption; Test using multimeter within circuit Analysis of power consumption; Test using multimeter within circuit Analysis of unit assembly; Demonstration of instrument disassembly Test of shortened operational cycle; Analysis of instrument stability of control/drift over 3 days Thermal analysis of the surrounding environment; Testing instrument in temperature-controlled environment (BioServe) Page 137 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 12.2.0 Component Functional Verification and Test The sensors that are to be used (e.g. temperature and pressure sensors) will need to be calibrated with known calibration laboratory instruments. This will ensure that the readings taken will be sufficiently accurate. Each powered component will also need to be tested for functionality prior to integration into each subsystem. This includes the motors for the mixers, as well as every TEC, strip heater, and peristaltic pump used. The safety mechanisms, such as the pressure release valve on the autoclave, the sensors, and any and all switches used must also be tested in the laboratory to detect any possible faulty or broken components. These will need to be tested again once installed in the instrument to make sure that all of the proper connections are made and the desired voltages are achieved. 12.3.0 Subsystem-Level Verification and Test PDD 4.11 requires that the reaction sample be steam sterilized up to three times in order to achieve thorough sterilization. To verify that the autoclave and the Data Acquisition and command software can handle repetitive cycles, the autoclave will be run three times prior to integration into the entire system. The temperature limits must be reached in order to verify that the autoclave is successful. Once completed, a portion of the sterile sample will be culture tested at BioServe to ensure that there are no living organisms left in the sample. The DAQ and control software will also be tested prior to system-level integration using a simulator provided by BioServe that allows the user to set an analog input voltage and to display the digital and analog output voltages. This simulator allows testing of the software without any instrument hardware, which permits parallel development of the software and hardware. The DAQ and control software will also be tested during the autoclave testing. The temperature and pressure sensors within the autoclave will be connected to the DAQ during testing in order to collect and store the heating and cooling data. The control software will be responsible for commanding the TEC and the strip heaters to heat or cool as necessary to achieve the appropriate temperatures within the autoclave. The ability of the software to provide caution, warning, and status signal will also be tested by setting a maximum temperature and/or pressure within the autoclave. When the autoclave approaches this maximum, a caution or warning signal should appear. If the autoclave exceeds the maximum, there will be an emergency shut-off. The mixer within each reaction chamber will also be tested prior to system-level integration as mentioned in section 11. As mentioned in Table 12-1, this will be through visual and video verification. The appropriate volume of the sample to be tested will be mixed with water and put in the reaction chambers with the sensors in place. The mixer will then be powered on and the tester will examine the chambers and verify that there is fluid movement around the sides of the chamber and that the amount of sedimentation will not affect the sensor readings. The solution does not have to be completely homogeneous, but there should be enough of the sample moving around the sensors to achieve accurate readings. The sensors do not need to Page 138 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 take readings during this mixing test, although they must be in place to maintain the seal around the holes in the reaction chamber. 12.4.0 System-Level Verification and Test Once the entire instrument is assembled, system-level testing will begin. The instrument will be placed in BioServe’s temperature-controlled testing environment. For a worst-case test, this environment will be set to -10°C. The desired sample will be placed in the autoclave through the top of the chassis and the autoclave cap will be screwed on. Then the autoclaving process will begin. Since the autoclave repetition requirements will have been verified prior to system-level testing, only one autoclave cycle will be performed. Once this cycle is complete, the software will alert the experimenter who will then open the butterfly valve. The reagent water will flush through the autoclave pushing the sample into the reaction chambers. Once the sample is in the reaction chambers, the mixing and data acquisition for the experiment will begin. PDD requirement 4.29 states that the experiment takes place for 14 days. The MiDAs instrument, however, will only be tested for three complete days while varying the temperature in BioServe’s testing environment. This verifies that the command software and the thermal control in the MiDAs environmental chamber satisfy the temperature requirement (4-37°C). Three full days is sufficient to verify the requirements set forth in the PDD. The MiDAs instrument will be tested under three ambient conditions during the testing phase to ensure that steady state can be reached. There are two worst-case conditions that must be tested. The first is maintaining the reaction chamber environment at 37°C with an ambient temperature of -10°C, which is the low end of the potential operational environment outlined in PDD requirement 4.30. The other worst-case to test is maintaining the chambers at 4°C with an ambient temperature of 40°C. The third test is a nominal case, which will be controlling the environmental chamber between 4° and 37°C with an ambient temperature of about 20°. For these conditions, the current and power draw will be measured using a multimeter to ensure that the MiDAs environmental set points can be maintained within the required accuracy and stability. If the system is stable for three days, then it is assumed stable over 14 days. During the 3-day testing period, the sensor drift will also be monitored. If the drift is very small in this time frame, then the drift over two weeks will not be detrimental. The DAQ memory will also be investigated at the end of the testing phase. If it is possible that the memory would be full after two weeks, then the data may have to be downloaded from the onboard computer periodically throughout the experiment. Page 139 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 13.0 Project Management Plan Author: Elizabeth Newton Page 140 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 13.1.0 Organizational Responsibilities MiDAs Project Management Fabrication Design Document Verification and Testing Project Manager Elizabeth Newton Lead Fabrication Engineer Dave Miller Design Engineer Chuck Vaughan Systems Engineer Shayla Stewart Assistant Project Manager Ted Schumacher Assistant Fabrication Engineer Sameera Wijesinghe Design Engineer Jeff Childers Software Engineer Steven To Assistance as Needed from Team Assistance as Needed from Team Assistance as Needed from Team Figure 13-1 Organizational Responsibilities Figure 13-1, above, shows organizational responsibilities for the fabrication and testing phase of the project. It is broken up into four components: project management, fabrication, design document work, and verification and testing. The responsibilities of each team are summarized in the work breakdown structure in the next section. Page 141 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 13.2.0 Work Breakdown Structure MiDAs Project Management Fabrication Design Document Verification and Testing Scheduling Fabricating Chambers TRL 6-7 Design Subsystem Integration and Testing Organizing Meetings Fabricating Chassis and Supports Preliminary Drawings of Designs System Integration and Testing Interface with Customer Integrating Electrical and Mechanical Systems Creating Design Document Creating Testing Software Maintaining Technical Drawings Creating Instrument Software Figure 13-2 Work Breakdown Structure Figure 13-2 shows the work breakdown structure. Each team is responsible for various tasks, which are outlined in the figure and explained below. The responsibility of the project management team is to ensure that the rest of the project runs smoothly. This includes duties such as scheduling, organizing and running meetings, and interfacing with the customer. These tasks will be performed by the project manager and the assistant project manager. The fabrication team is in charge of the actual creation of the parts. This includes maintaining and updating SolidWorks models and drawings, as well as machining the parts. These tasks will be primarily performed by the fabrication engineer and the assistant fabrication engineer, but the rest of the MiDAs team will assist with less complicated parts. The MiDAs team also has access to BioServe’s machine shop, which can fabricate some parts as well. The design document team is in charge of upgrading the field instrument to be more Marsapplicable. These upgrades will not be implemented, but will be included in a design Page 142 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 document to be delivered to the customer. This document will provide design suggestions to future teams working on this project on how to make the instrument more automated and able to survive in a Mars-like environment. The whole MiDAs team will work on this document as necessary. Verification and testing includes development of testing and integration plans as well as actually assembling and testing the system and subsystem. The team must show that each requirement in the PDD is verified appropriately. All members of the MiDAs team will assist in the integration and testing of the instrument. 13.3.0 Schedule Figure 13-3 Schedule for Spring Semester Figure 13-3, above, shows the schedule for the MiDAs team for the second phase of the project. This phase will include the fabrication, integration, and testing of the MiDAs project. There are three primary tasks for the group as a whole. First, all parts will be ordered during Winter Break and the first two weeks of the semester. Priority will be given to the components that go into fabricating the autoclave and test chambers, since these are machined first. Second, the entire team will assist with the component integration. Third, everyone will work on the users’ manual. Creating the manual spans about seven weeks of the second semester, and will be written concurrently with the integration of the components. This will help ensure that users’ manual is as clear and precise as possible. Page 143 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 The fabrication team will be in charge of manufacturing all the components for the project. In order to facilitate integration and iron out problems early, each “path” of the instrument will be manufactured separately. This means that one autoclave, one reaction chamber, and the attachments between them will be made first, followed by the second set. The fabrication team will also help with the electrical connections and integration towards the end of the semester. The testing and verification teams will be in charge of assembling and integrating the various components. They will then test the components according to the testing and verification plan. The testing team will also be in charge of writing LabView code both for testing and for actual instrument functionality. 13.4.0 Specialized Facilities and Resources The MiDAs team has access to several special facilities and resources. In addition to the aerospace machine shop and electronics shop, BioServe is providing assistance in many ways. BioServe is providing the team with matching funds, bringing our total budget to $8,000. BioServe can also provide small parts, such as screws, wires, and spare materials if they are available. BioServe also has its own machine shop, which is run by Don Geering. Mr. Geering is available to help the fabrication team by making parts if we run short on time, or by fabricating delicate or complicated parts, such as the sensor ports on the reaction chambers. For testing purposes, BioServe has several facilities available for the MiDAs team. They have a temperature-controlled testing chamber where the instrument can be tested in variable-temperature environments to ensure that the thermal controls function properly. They also have a biological lab available to test the sterility of the soil transportation. Also, BioServe can provide a clean room to assemble the sterile components. Page 144 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 13.5.0 Overall Budget The overall budget for MiDAs is shown in Table 13-1. This table includes all known parts. The numbers shown are raw and do not include a safety factor. The total budget is just over $4,550, which is $3,450 below our total allotted budget. Item Thermal Control Melamine Insulation Strip Heater Thermoelectric Cooler (TEC) Heat Sink Sensors Mechanical Quantity Cost 1 (24”x48”x2”) 7 $50 $237 4 $107 Melcor HX6-201-L-M 4 $46 Temperature National SA1-RTD 6 $300 Pressure Omega PX139 4 $340 ISE Package Tufts -- 2 -- Ultem 1000 8686K81 $155 6384K44 1 (24”x2” rod) 2 (12”x2.5” rod) 2 (48”x48”x.0625”) 2 Rotary Shaft Ring Seal Pumps McMasterCarr McMasterCarr McMasterCarr McMasterCarr McMasterCarr Instech 9562K41 2 $7 P625/275.133 2 $690 Motors Micromo 1224 2 $600 Butterfly Valve McMasterCarr Dimond Systems Kontron 4820K31 2 $174 DMM-37X-AX 2 $480 MOPSlcdLX 1 $450 PA75CC 2 $25 WTC3243 4 $348 TOTAL $4554 316 Stainless Steel Aluminum Bearing Computer/DAQ Table 13-1 Overall Budget Vendor Vendor Part Number McMaster86145K27 Carr Minco HK5544R33.1L1 2B Melcor CP-0.8-127-06L DAQ Embedded CPU Mixer Controller Thermoelectric Controller Apex Microtechnology Apex Microtechnology 89325K673 89015K53 $300 $230 $15 Page 145 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.0 Appendix A: Mechanical Design Drawings for the parts that will be manufactured are shown on following pages. 14.1.0 Mechanical Drawing Tree Page 146 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.2.0 Sterilization Chamber Lid MiDAs-SC-101-01 – Sterilization chamber lid - SolidWorks part and drawing by DM Page 147 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.3.0 Sterilization Chamber Body MiDAs-SC-201-01 – Sterilization chamber body - SolidWorks part and drawing by DM Page 148 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.4.0 Sterilization Chamber Bottom MiDAs-SC-401-01 – Sterilization chamber bottom - SolidWorks part and drawing by DM Page 149 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.5.0 Sterilization Chamber Assembly MiDAs-SC-A-000-01 – Sterilization chamber assembly - SolidWorks part and drawing by DM Page 150 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.6.0 Reaction Chamber Cap MiDAs-RC-P-301-01 – Reaction chamber cap - SolidWorks part and drawing by DM Page 151 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.7.0 Reaction Chamber Base MiDAs-RC-P-401-01 – Reaction chamber base - – SolidWorks part and drawing by DM Page 152 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.8.0 Reaction Chamber Body MiDAs-RC-P-101-01 – Reaction chamber body – SolidWorks done by JF and PK at BioServe Drawing done by DM based on that part Page 153 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 14.9.0 Reaction Chamber Assembly MiDAs-RC-A-000-01 – Reaction chamber assembly – body from SolidWorks from BioServe, cap, bottom and overall drawing by DM Page 154 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 14.10.0 MiDAs December 18th, 2006 Reagent Water Chamber Body MiDAs-WC-P-101-01 – Reagent water chamber body – SolidWorks part by SW, drawing by DM Page 155 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 14.11.0 MiDAs December 18th, 2006 Water Chamber Cap MiDAs-WC-P-102-01 – Water chamber cap - – SolidWorks part by SW, drawing by DM Page 156 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 14.12.0 MiDAs December 18th, 2006 Top Support Shelf MiDAs-OC-P-101-01 – top shelf holding sterilization chambers - – SolidWorks part by SW, drawing by DM Page 157 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis 14.13.0 MiDAs December 18th, 2006 External Case MiDAs-OC-P-102-01 – External case, basic dimensions only - – SolidWorks part by SW, drawing by DM Page 158 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.0 Appendix B: Electrical Design 15.1.0 Electrical Schematic Tree Subsystem/Item Drawing Number Electrical Block Diagram MiDAs-OS-A-200-01 Power Subsystem Power Supply Power Distribution MiDAs-OS-A-210-01 MiDAs-OS-P-211-01 MiDAs-OS-P-212-01 Sensor Subsystem Wire Harness Pressure Sensors Temperature Sensors Control Subsystem Mixer/Control 1 Mixer/Control 2 Autoclave TEC/Control 1 Autoclave TEC/Control 2 RC TEC/Control 1 RC TEC/Control 2 Wire Harness Fan Switch Board Strip heaters LEDs Wire Harness Water Pump Data Acquisition and Control Computer Analog input Digital input Analog Output MiDAs-OC-A-220-01 Drawing Name Drawn by CV Power System CV Sensor Schematics CV Control Schematics CV MiDAs-SC-P-221-01 MiDAs-SC-P-222-01 MiDAs-SC-P-223-01 MiDAs-OS-A-230-01 MiDAs-SC-P-231-01 MiDAs-SC-P-232-01 MiDAs-SC-P-233-01 MiDAs-SC-P-234-01 MiDAs-SC-P-235-01 MiDAs-SC-P-236-01 MiDAs-SC-P-237-01 MiDAs-SC-P-238-01 MidAs-OS-A-240-01 Switch Board Layout CV Computer Interface CV MiDAs-SC-P-241-01 MiDAs-SC-P-242-01 MiDAs-SC-P-253-01 MiDAs-SC-P-254-01 MidAs-OS-A-250-01 MiDAs-SC-P-251-01 MiDAs-SC-P-252-01 MiDAs-SC-P-253-01 MiDAs-SC-P-254-01 Page 159 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.2.0 Overall Electrical Schematic Electrical Schematic 110VAC Power Source 12V +5V Power Conditioning/ Protection 12V Power Distribution/ Switching CPU Communication (USB) T Dig.Out T P Data Acquisition/ Control Autoclave 1 T AHC2 Temp Control/ Power cut off Autoclave 2 P Water Pump L1 Control Chamber AHC 1,2: Autoclave heater/cooler 1,2 An.In: Analog input An.out: Analog output T=Temperature Sensor P=Pressure Sensor Dig.Out: Digital Output Ambient AHC1 Temp control/ Power Cut off Reagent Water Chamber Control Switch An.In An.out P T P L2 Test Chamber T T Speed control M1 Electrochemical Sensors(12) M2 Speed control IHC: Incubator Heater/Cooler L 1,2: Light 1,2 M1,2: Mixer 1,2 Temp control IHC Reaction Chamber Environment Page 160 of 196 1 TEC AC 2 TEC AC 1 TEC RC 2 TEC RC 1 Mixer 2 Mixer 2 Control 2 TEC 1 TEC Autolcave x 4 x 2 x 4 x 2 x 4 Autoclave 1 Control RC x 2 x 4 x 2 x 3 x 2 RC 1 Control Mixer x 3 x 2 2 Control Mixer H2 Autoclave LED's H1 Autoclave Pump1 2 Pump 6 x Output Analog x 1 1 x x output Digital x 3 6 Board Switch 2 2 x 2 x 2 x 2 x 2 Input Analog x 0 1 x 0 2 Sensors x 2 Computer Distribution Power Supply Power Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.3.0 Electrical block diagram Page 161 of 196 Diagram Sensor D Sig G 5 6 Page 162 of 196 D N D N R 4 N VS + 3 G PX139 2 G D N G 1 AP2 7 8 PX139 9 0 1 R 1 1 2 1 ° t ° K 0 1 t K 1 0 Thermistor 6 R P R K K 0 1 D N G 0 1 Sig VS + Q A D PX139 Thermistor 5 R K 0 1 R K 0 1 ° t Sig VS + Thermistor 4 R K 0 1 K 0 1 ° t D N G AP1 Thermistor 3 R PX139 K 0 1 K 0 1 ° t R Thermistor 2 R K 0 1 Sig VS + K 0 1 ° t Thermistor 1 R P A +5VDC D N G D N G F u 0 0 1 Cap Zener D 1 .1uF .22uF ? C ? D Earth Cap Cap D N G 1 15.5.0 Sensor Diagram ? C ? C V 2 1 + Breaker Circuit SW-SPST T U O N I 2 3 Supply Power Amps 5 +5VDC LM340-XX +12VDC Switch off On ? U Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.4.0 Power Diagram Motor Pump M ? B 5 2 6 P System Control D N G CLR 8 MI + 7 MI - 6 PI DC 5 PG 4 PG 1 3 +V(ref) RWC 2 SCI R -V(ref)1 Pump Peristaltic 5 6 7 8 9 +12VDC +5VDC G G D N G .22uF 5 Cap ? C 2.27MOhm G PA75CC 1.5K .22uF B A O D N G D N G D N G D N G K 1 H G Tap Res LED2 LED1 2 H R ? D N D N Page 163 of 196 K 5 K 5 K 5 K D N G D N G D N 1.5K 1.5K 1.5K .22uF D 4 N R 3 D 2 N G D N G 1 Computer Cap Cap Vs + ? C ? C IAB + 4.9K 4.9K Mixer1 Vs - M P R P R ? B IAA + D N G 33.3K 33.3K IAA - 2.27MOhm 2.27MOhm A A O I R I R .22uF Control Motor Cap K 1 ? C Res3 Res3 2.27MOhm R R L L C C 8 9 1 1 1 1 1 7 0 1 2 3 4 6 5 4 3 2 1 8 9 1 1 1 1 1 7 0 1 2 3 4 6 5 4 3 2 1 F R I R D N G WTC3243 WTC3243 CONTROLLER TEC CONTROLLER TEC PA75CC K 0 2 K 0 2 B A O D N G Vs + RBIAS RBIAS IAB + Mixer1 Vs - M ? B IAA + IAA - A A O ° t K 0 1 ° t D N K 1 G D N G Control Motor Thermistor IHC1 K 0 1 IHC2 K 1 K 1 Thermistor Res3 Res3 F R I R Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.6.0 Control Diagrams Motor Pump M ? B 5 2 6 P System Control D N G CLR 8 MI + 7 MI - 6 PI DC 5 PG 4 PG 3 +V(ref) K 1 2 SCI Tap Res -V(ref)1 D N G ? R Pump Peristaltic 1 2 3 4 5 6 7 8 9 +12VDC Computer +5VDC D N G D N G Page 164 of 196 K 5 K 5 K 5 K 5 D N G D N G .22uF .22uF 1.5K 1.5K 1.5K 1.5K Cap Cap ? C ? C 2.27MOhm 2.27MOhm 4.9K 4.9K P R P R 33.3K 33.3K I R I R R R L L C C 8 9 1 1 1 1 1 7 0 1 2 3 4 6 5 4 3 2 1 8 9 1 1 1 1 1 7 0 1 2 3 4 6 5 4 3 2 1 WTC3243 WTC3243 CONTROLLER TEC CONTROLLER TEC K 0 2 K 0 2 RBIAS RBIAS ° ° t t K 0 1 K 0 1 Thermistor IHC2 Thermistor IHC2 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 D N V 2 1 + V 5 6 3 5 3 D N G + 0 G 8 3 7 3 4 9 V 5 + 3 V 2 1 + D N G 4 3 Distribution Power V 5 + 2 3 V 2 1 + 0 3 D N G 8 2 V 2 1 + 6 2 D N G 4 2 V 2 1 + 2 2 D N G 0 2 V 2 1 + 8 1 7 1 Sig D N G 6 1 5 1 Sig V 2 1 + 4 1 3 1 Sig D N G 2 1 1 1 Sig V 2 1 + 0 1 9 Sig D N G 8 7 Sig V 2 1 + 6 5 Sig D N G 4 3 Sig V 2 1 + 2 1 Sig Items Switched Board Switch Output Digital Diagram Wire System D N G D N G V 2 1 + V 2 1 + Computer Supply Power Sig D N G Sig V 5 + D N G AT2 V 2 1 + Sig Pump D N G D N G V 5 + V 2 1 + AT1 Heater2 Sig D N G D N G V 2 1 + V 5 + Heater1 Sig D N G D N V 2 1 + V 5 Pump T Sig Sig C + V 5 + T G D N G A Sig Sig D N G D N G Sig V 2 1 + Sig V 5 + D N G D N G Sig V 2 1 + D N G V 5 + D N G V 2 1 + V 5 + TEC4 T T D N G Sig Sig V 2 1 + Sig D N G D N G Sig D N G V 5 + V 2 1 + D N G V 5 + Sig V 2 1 + D N G TEC3 T R Sig V 5 + V 5 + D N G Sig Sig D N G V 2 1 + D N G D N G V 2 1 + Sig V 5 + V 5 + D N G Sig TEC2 P R V 2 1 + D N G V 5 + V 5 + Sig V 5 + D N G D N G D N G Sig V 2 1 + V 2 1 + V 5 + D N G V 5 + V 5 + TEC1 AP2 D N G Sig Sig V 2 1 + Sig D N G D N G Sig D N G V 5 + V 2 1 + D N G V 5 + Sig V 2 1 + D N G Mixer2 AP1 Sig V 5 + Sig D N G Sig Input DAQ D N G V 2 1 + D N G V 2 1 + V 5 + inputs Control Mixer1 P A Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 15.7.0 Wire Diagram 15.8.0 Switch Board Page 165 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 16.0 Appendix C: Software 16.1.0 Software Tree AIn = Analog Input: Acquires pressure and temperature data DBit Out = Digital Bit Out: toggles output high or low to control the switch board Err Msg = Error message: displays error message if output is not configured right To Eng = Converts binary inputs from levels to voltage level ToEngArray= Converts array of binary inputs to voltage level = Autoclave temperature/pressure.vi = Elapse Timer: Counts amount of time elapsed after specific case = Time Delay: Waits specified time before taking next sensor data = Write File: Writes data to measurement file Page 166 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 16.2.0 Software Prototype Page 167 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.0 Appendix D: Data Sheets Page 168 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.1.0 Motor Page 169 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.2.0 TEC Page 170 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 171 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.3.0 Motor Control Page 172 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 173 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 174 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 175 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.4.0 Pressure Sensor Page 176 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.5.0 TEC Controller Page 177 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 178 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 179 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 180 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 181 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 182 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 183 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 184 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 185 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 186 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 187 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 188 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.6.0 Peristaltic Pump Page 189 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 190 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 191 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.7.0 Voltage Regulator Page 192 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 17.8.0 CPU Page 193 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 Page 194 of 196 Fall Final Report ASEN 4018 – Senior Projects I: Design Synthesis MiDAs December 18th, 2006 18.0 Appendix E: References Cengel, Yunus. Introduction to Thermodynamics and Heat Transfer. McGraw-Hill. University of Nevada, Reno. 1997 Gilmore, David. Spacecraft Thermal Control Handbook. Aerospace Press. El Segundo, California. 2002 Mankins, John C. “Technology Readiness Levels.” April 6, 1995. <http://ipao.larc.nasa.gov/Toolkit/TRL.pdf.> www.dimondsystems.com www.kontron.com www.matweb.com www.mcmaster.com www.melcor.com www.minco.com www.omega.com www.sonaer.com www.sonozap.com www.claybornlab.com www.thomasnet.com www.coleparmer.com www.taycoeng.com www.watlow.com www.aerogel.com Page 195 of 196