CM5201 Expt 1
advertisement

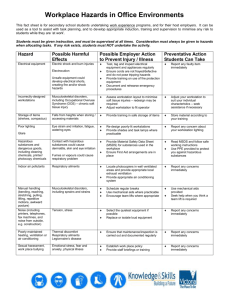
DEPARTMENT OF CHEMISTRY TEACHING LAB EXPERIMENT RISK ASSESSMENT FORM This form must be completed jointly by the Lab Officer in charge and the Lecturer in charge. A hardcopy of the completed form should be kept in a file together with the Project Risk Assessment. Name of Lecturer in Charge Name of Lab Officer in Charge Module / Expt No. Ms Frances Lim CM 5201 Expt 1 Activity being assessed: Synthesis of N-phenylmaleimide Known or expected hazards associated with the activity: Hazards of reagents, solvents and known reaction products. State each substance and the approximate amounts to be used/produced. Maleic Anhydride,0.98g - Harmful if swallowed, inhaled or absorbed through the skin. Corrosive - causes burns. Irritant. Aniline,0.91ml - Causes irritation to skin, eyes and respiratory tract. Combustible liquid and vapour. Diethyl Ether,8ml - Extremely flammable liquid and vapour. Causes irritation to skin, eyes and respiratory tract. Affects central nervous system. Acetic Anhydride,3.4ml - Corrosive. Flammable liquid and vapour. Water reactive. Sodium Acetate,0.41g - May cause irritation to skin, eyes and respiratory tract. Acetone for washing - Extremely flammable liquid and vapour. Eye and respiratory irritant. May cause drying and cracking of the skin. N-phenylmaleimide (product) - Toxic. Eyes, skin and respiratory irritant. May be harmful if swallowed. Incompatible materials (special precautions): Maleic Anhydride - Incompatible with water, strong oxidizing agents, alkali metals, strong bases, amines, most common metals, polymerization catalysts and accelerators. Aniline - Strong acids and strong oxidizers, solutions of iron, zinc, aluminum and alkalis. Ignites spontaneously in the presence of red fuming nitric acid, and with sodium. Diethyl Ether - Can react dangerously with acetyl peroxide, liquid oxygen, bromoazide, chlorine, and strong oxidizers such as nitrates. Avoid heat, flame, other sources of ignition, and exposure to light, air. Acetic Anhydide - Water, steam, mineral acids, oxidizing materials, alcohols, or amines may cause violent reaction. Contact with strong caustics will cause violent reaction and spattering. Corrosive to copper, brass, bronze, and iron. Sodium Acetate - Nitric acid, fluoride, potassium nitrate, strong oxidizers. N-phenylmaleimide - Strong oxidizing agents, strong bases. The risk of injury and its severity likely to arise from these hazards: Maleic Anhydride - Repeated inhalation may cause chronic bronchitis of the asthmatic type. Page 1 of 4 Printed on: 07 March 2016 Repeated skin contact may lead to dermatitis or sensitization. Aniline - Affects blood, cardiovascular system, central nervous system, liver and kidneys. Diethyl Ether - General anesthesia by inhalation can occur. Continued exposure may lead to respiratory failure or death. Early symptoms include irritation of nose and throat, vomiting, and irregular respiration, followed by dizziness, drowsiness, and unconsciousness. Acetic Anhydride - Vapour causes respiratory tract irritation and severe eye irritation. Sodium Acetate - May cause irritation to the respiratory tract. Symptoms may include coughing, sore throat, labored breathing, and chest pain. N-phenylmaleimide – Potential health effect - No data found. Acetone: Hazardous if inhaled or ingested. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. Inflammation of the eye is characterized by redness, watering, and itching. Who is at risk? Students handling the chemicals as well as staff in the vicinity. Measure to be taken to reduce the level of risk: Proper PPE must be worn. Solvent dispensers are provided to minimise contact with solvents. Refer to prepared risk assessment on Use of Glassware. Please refer to PSSO Safety Information Centre website on safety measures: http://www.chemistry.nus.edu.sg/PSSO/Safety.htm. Training prerequisites: Please refer to Completed Risk Assessment on Common Activities: http://www.chemistry.nus.edu.sg/PSSO/Safety/Risk/risk.htm#Common. Level of risk remaining: Low Emergency action if : Spill: Ventilate area. Remove all sources of ignition. Wear appropriate PPE. Isolate hazard area. Keep unnecessary and unprotected personnel from entering. Contain and recover liquid when possible or absorb with an inert material (eg Vermiculite, dry sand, earth), and place in a chemical waste container. Fire: Use ABC extinguisher available in the lab. Water spray may be used to keep fire exposed containers cool to prevent pressure build-up, auto ignition or explosion, protect personnel. Is the experiment suitable for out-of-hours operation ? Yes No References if any: http://www.jtbaker.com/msds/englishhtml/a0338.htm Page 2 of 4 Printed on: 07 March 2016 http://www.jtbaker.com/msds/englishhtml/A0446.htm http://www.jtbaker.com/msds/englishhtml/m0364.htm http://www.jtbaker.com/msds/englishhtml/a6660.htm http://www.jtbaker.com/msds/englishhtml/s2666.htm http://www.jtbaker.com/msds/englishhtml/E2340.htm http://www.coleparmer.com/catalog/Msds/38728.htm Signature of Lab Officer in Charge:……………………………………………………………….. Date:………………………… Signature of Lecturer in Charge:………… …………………………………….. Date:… …………………….. Prepared Risks Assessments for standard equipment and operation are with the kind permission of Dr. Ken MacNeil, School of Chemistry, University of Bristol. Page 3 of 4 Printed on: 07 March 2016 Activity being assessed: Note any activity to be used which entail risk (e.g. use of glass vacuum apparatus, high pressures, high voltage, radiation, high temperatures). Give reference to any special protocols to be followed, and if appropriate attach copies to the risk assessment form. State any additional precautions taken to minimise risk. Known or expected hazards associated with the activity: FOR EACH CHEMICAL, read the MSDS and note:a) Particular hazards (e.g. highly toxic, carcinogenic, corrosive, flammable, pyrophoric, explosive, volatile, dust hazard). Note any dangerous combinations of properties (e.g. volatile and toxic). b) Requirements for safe handling (e.g. fume cupboard, inert atmosphere, low temperature). c) How to dispose of residuals Dispose to drain, with water dilution Neutralise, then to drain with suitable dilution To flammable liquid waste receptacle To non-flammable liquid waste receptacle Keep for recovery/recycling Keep for special disposal later (e.g. heavy metals) Double bag and dispose to dry waste Special procedure (specify) Incompatible materials (special precautions) Note any dangerously incompatible materials and hazards arising from contact of any reagents and substances used with common materials such as paper, benches, hoses, etc. Measures to be taken to reduce the level of risk Include hazards of previously unknown products. Location of work – laboratory, open bench, fume cupboard Level of risk remaining: Likelihood and consequences of any accident or unforeseen events whilst carrying out the activity. When this has been done, choose the appropriate procedure:a) Close supervision and/or attendance of trained first-aider needed. b) Specific approval of supervisor needed. c) Training is needed prior-to or during the operations specified. d) Training is complete and only general laboratory competence required. e) No risk perceived. Emergency action: a) Any special requirements to deal with accidental spillage or leakage. b) What to do in the event of accidental exposure (skin contact, inhalation, etc.). Page 4 of 4 Printed on: 07 March 2016