q4s08
advertisement

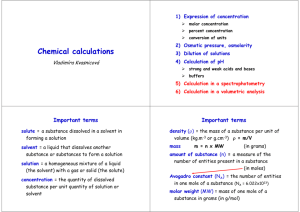
CH302 Quiz 4 What is the pH of 2.0 * 10-9 M Ba(OH) 2? 1. 7.02 correct 2. 5.30 3. 8.70 4. 8.40 5. 5.60 What is the buffer capacity of 100ml of 0.1M HClO2 and 100ml of 0.2M NaClO2? 1. 0.01 mol of OH- and 0.02 mol of H+ correct 2. 0.02 mol of OH- and 0.01 mol of H+ 3. 0.1 mol of OH- and 0.2 mol of H+ 4. 0.2 mol of OH- and 0.1 mol of H+ 5. 10 mol of OH- and 20 mol of H+ Which of the following mixtures would produce a buffer? I) 100ml of 0.1M NH4Cl + 75ml of 0.1M NaOH II) 100ml of 0.1M NH3 + 100ml of 0.5M HCl III) 100ml of 0.1M NaCOO + 50ml of 0.2M HCl IV) 100ml of 0.1M HClO2 + 100ml of 0.1M NaClO2 V) 100ml of 0.1M NH3 + 75ml of 0.1M NaOH 1. I and IV only correct 2. I, IV, and V only 3. I, II, III, and IV only 4. II and III only 5. I only What is the final pH of a solution containing 100ml of 0.5M HNO2 and 100ml of 0.1M NaNO2 after 0.03 mol of NaOH are added? The pKa is 3.37. 1. 3.67 correct 2. 1.02 3. 3.03 4. 10.33 5. 3.37 What is the pH of 0.5 M NaHCO3? The Ka1 is 4.266 * 10-7 and the Ka2 is 5.623 * 10-11. 1. 8.31 correct 2. 5.69 3. 3.34 4. 5.28 5. 10.67 The figure below shows the titration of a monoprotic weak acid with a strong base. The pH at point I is equal to the ________ and the pH at point II is _______ 7. 1. pKa of the acid, greater than correct 2. pKa of the acid, less than 3 pH of the acid, greater than 4. pH of the acid, less than, 5. pKa of the acid, equal to What is the pH when 100ml of 0.1 M HCl is titrated with 50ml of 0.2 M NaOH? 1. pH = 7 correct 2. pH > 7 3. pH < 7 4. the pKa of HCl needs to be provided to answer this question 5. the pKb of NaOH needs to be provided to answer this question What is the pH when 100ml of 0.1 M NH3 is titrated with 50ml of 0.2 M HCl? The pKa of NH4Br is 9.25. 1. 5.21 correct 2. 5.63 3. 5.12 4. 2.96 5. 8.79