BELLARMINE COLLEGE
advertisement
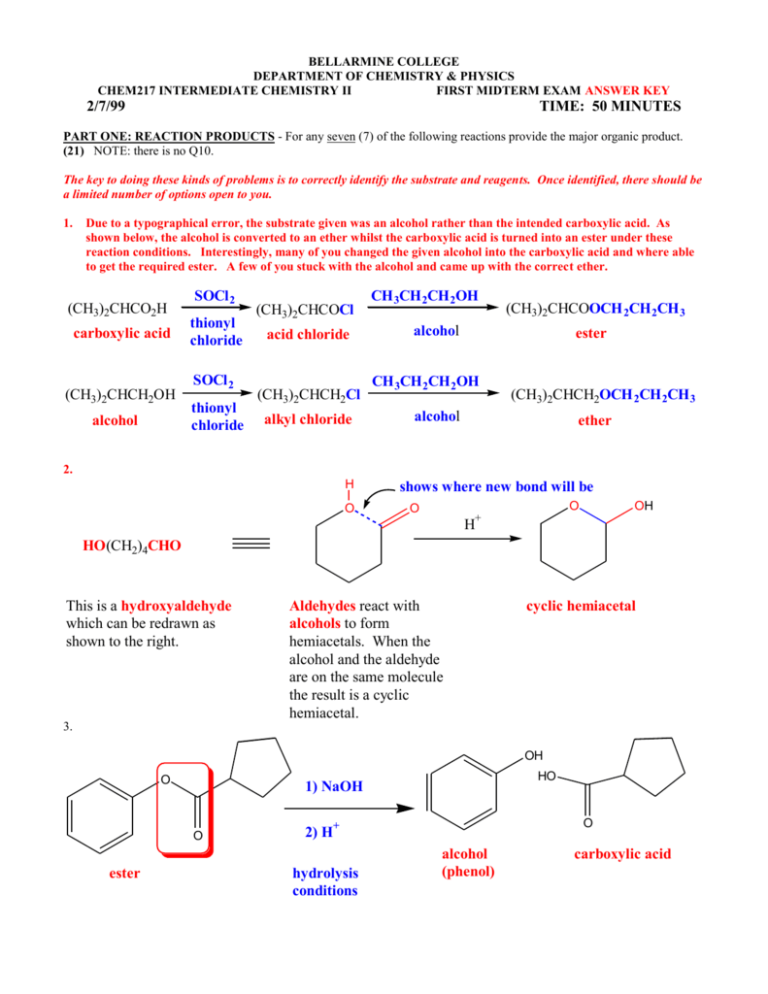
BELLARMINE COLLEGE DEPARTMENT OF CHEMISTRY & PHYSICS CHEM217 INTERMEDIATE CHEMISTRY II FIRST MIDTERM EXAM ANSWER KEY 2/7/99 TIME: 50 MINUTES PART ONE: REACTION PRODUCTS - For any seven (7) of the following reactions provide the major organic product. (21) NOTE: there is no Q10. The key to doing these kinds of problems is to correctly identify the substrate and reagents. Once identified, there should be a limited number of options open to you. 1. Due to a typographical error, the substrate given was an alcohol rather than the intended carboxylic acid. As shown below, the alcohol is converted to an ether whilst the carboxylic acid is turned into an ester under these reaction conditions. Interestingly, many of you changed the given alcohol into the carboxylic acid and where able to get the required ester. A few of you stuck with the alcohol and came up with the correct ether. (CH3)2CHCO2H carboxylic acid (CH3)2CHCH2OH alcohol SOCl 2 thionyl chloride SOCl 2 thionyl chloride (CH3)2CHCOCl acid chloride (CH3)2CHCH2Cl alkyl chloride CH 3CH 2CH 2OH (CH3)2CHCOOCH 2CH 2CH 3 alcohol ester CH 3CH 2CH 2OH (CH3)2CHCH2OCH 2CH 2CH 3 alcohol ether 2. H O shows where new bond will be O O OH + H HO(CH2)4CHO This is a hydroxyaldehyde which can be redrawn as shown to the right. Aldehydes react with alcohols to form hemiacetals. When the alcohol and the aldehyde are on the same molecule the result is a cyclic hemiacetal. cyclic hemiacetal 3. OH O O ester HO 1) NaOH O 2) H+ hydrolysis conditions alcohol (phenol) carboxylic acid 4. H+ (CH3)3CCH2CH2OH (excess) + C6H5CH2CO2H alcohol carboxylic acid (CH3)3CCH2CH2O2CCH2C6H5 ester OR O OH HO Fischer esterification excess alcohol used to drive equilibrium to product side. H+ O (+ H2O) O 5. via H+ + HOCH2CH2CH2OH O O diol O ketone hemiacetal OH O diols are quite often used to protect aldehydes and ketones by converting them to cyclic acetals. cyclic acetal HO alcohol 6. CH3CH2CH2Cl + NaOH o 1 alkyl chloride substrate hydroxide nucleophile CH3CH2CH2OH + NaCl o 1 alcohol chloride product leaving group This is an example of a SN1 reaction 7. CH3COCl + acid chloride OR carboxylic acid salt O O Cl 8. CH3CO2-Na+ -O CH3CO2COCH3 (+ NaCl) carboxylic acid anhydride O O O H2O/H+/ C6H5CN C6H5CO2H hydrolysis conditions nitrile (+ NH3) via C6H5CONH2 carboxylic acid amide O OR O N C NH2 OH -NH3 + H2O 9. O O O O + + HO O HO O carboxylic acid anhydride alcohol (phenol) ester carboxylic acid 11. OH OCH 3 H+ + CH3OH (+ H2O) OCH 3 hemiacetal OCH 3 alcohol acetal 12. OH H+ O (+ CH3OH) OCH 3 hemiacetal ketone alcohol PART TWO: REACTION MECHANISMS - Provide a reasonable mechanism for either one of the following reactions. (14) 13. H H+ O O O O O OH2 + HO acetal OH H H + O O H H O O H+ O H O H protonated hemiacetal O H+ ketone 14. O O O H2O O O H+ O H H ester H H+ OH O O HO O OH O alcohol (phenol) H protonated tetrahedral intermediates H O OH H+ H O O OH OH carboxylic acid H H PART THREE: LE CHATELIER'S PRINCIPLE - Do each of the following questions. The equilibrium constant for the Fischer esterification of isopropyl alcohol (2-propanol) with acetic acid (ethanoic acid) is 2.35. (15) 15. Write the chemical equation for this equilibrium. H+ (CH3)2CHOH + CH3CO2H 2-propanol (isopropyl alcohol (CH3)2CHO2CCH3 + H2O ethanoic acid acetic acid 2-propyl ethanoate isopropyl acetate) 16. If the initial amounts of isopropyl alcohol and acetic acid used are 1.0 mol, how much isopropyl acetate (in mol) is produced at equilibrium? K = 2.35 = [ester][water]/[acid][alcohol] = x2/(1.0 - x)(1.0 - x) => 1.35x2 - 4.7x +2.35 = 0 and x = 2.88 or 0.61, (2.88 makes no sense) So, answer is 0.61 mol isopropyl acetate produced 17. If the amount of isopropyl alcohol used is increased 20-fold (no increase in amount of acetic acid used) how much ester will be produced at equilibrium? K = 2.35 = [ester][water]/[acid][alcohol] = x2/(1.0 - x)(20.0 - x) 1.35x2 - 49.35x + 47 = 0, therefore x = 35.6 or 0.98, (35.6 makes no sense) So, answer is 0.98 mol isopropyl acetate produced



