Qualitative analysis: TESTING FOR ANIONS
advertisement

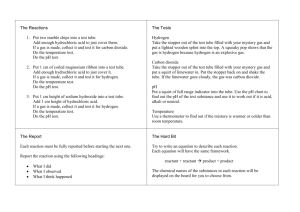
Grade 9 Integrated Science Practical # International School Manila Name: Date: QUALITATIVE ANALYSIS: TESTING FOR ANIONS AND GASES In this practical you will again practice following instructions and using your manipulative skills. You will also be assessed on how well you make and accurately record observations (Data Collection and Processing). Before coming to class you must decide on the clearest way to present your results. Make your data collection table and bring it with you to the lesson. You will not have time to record your results in rough. Introduction You will carry out some tests which can be used to identify anions (negative ions) and gases. Procedure Testing for anions Reagent Acidified Silver nitrate Anion (test solution) Chloride (Cl) (potassium chloride) Bromide (Br) (potassium bromide) Test Add a few drops of silver nitrate solution to approximately 1cm3 of each test solution. Leave your tubes in sunlight for at least 10 minutes Iodide (I) (potassium iodide) Lead nitrate Iodide (I) (potassium iodide) Add a few drops of lead nitrate solution (Toxic) to approximately 1cm3 of the test solution. Acidified Barium chloride Sulfate (SO42) (sodium sulfate) Add a few drops of barium chloride solution (Toxic) to approximately 1cm3 of the test solution. Hydrochloric acid Carbonate (CO32) (sodium carbonate) Add a few drops of dilute hydrochloric acid to approximately 1cm3 of the test solution. Testing for gases 1. Put a piece of shiny magnesium ribbon (approximately 2 cm in length) into a test tube. Add 2cm 3 of dilute hydrochloric acid. Place your thumb over the top of the tube (to stop any gas escaping). When you feel the gas inside exerting pressure on your thumb, remove thumb and immediately insert a lighted wooden splint into the tube. 2. Place manganese(IV) oxide powder (the volume of a pea) into a test tube. Add 2 cm3 of dilute hydrogen peroxide solution. Place your thumb over the top of the tube (to stop any gas escaping). When you feel the gas inside exerting pressure on your thumb, remove thumb and immediately insert a glowing wooden splint into the tube. 3. Place 1 cm3 of limewater into a boiling tube and put to one side. In a separate test tube add 1 cm 3 of dilute hydrochloric acid to a spatula full of calcium carbonate powder. Place your thumb over the top of the tube (to stop any gas escaping). When you feel the gas inside exerting pressure on your thumb, remove thumb and carefully pour the gas produced into the boiling tube. Stopper the boiling tube and shake. Grade 9 Integrated Science Practical # International School Manila Name: Date: Questions (You will work through your results with your teacher in order that you can answer the following) 1. 2. 3. 4. 5. 6. What are the names of the precipitates formed in the halide ion and the sulfate ion tests? Write balanced chemical equations for all of the reactions occurring in the anion tests. Identify the three gases produced and tested for in the gas tests. Write a balanced chemical equation for the reaction which produced hydrogen gas. What is the similarity between the carbonate ion test and the gas test 4? Write a balanced chemical equation for the reaction which produced oxygen gas.