The Heating Curve For Water
advertisement

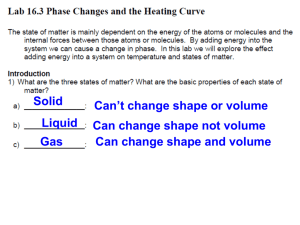
Haugen/Yost Name: __________________________ Date: ______________ Period: _____ Marks ___________ The Heating Curve For Water Materials Manager In charge of setting up materials and holding the thermometer during the experiment. Timer/Cleanup Manager In charge of timing during the experiment and notifying the recorders of “time” after every 30 seconds have passed. Also helps with cleanup. Recorder 1 In charge of recording the temperature every 30 seconds. Must be able to read the thermometer. Recorder 2 In charge of recording the “description of water” observations every 30 seconds. Is it ice, liquid, water, steam or a combination of these? PROBLEM: How does the temperature change if water is heated so that it changes phase from solid to liquid to gas? HYPOTHESIS: MATERIALS: thermometer, 600 ml beaker, ice, water, hot plate, watch PROCEDURE: 1. Turn on the hot plate so it can start warming up. 2. Add 150 ml of water and ½ cup of ice to the beaker. Place the beaker on the lab bench. 3. Place the thermometer in the mixture. Be careful with the thermometer. Do not let go of it. Hold it by the string or plastic piece at the top and hold it above the bottom of the beaker for the entire experiment. Keep the thermometer in the water at all times. Hold it there for 3 minutes before starting observations. 4. After 3 minutes take your first observation. Reset the stopwatch. Label the time for this observation as 0.0 minutes. 5. Place the beaker on the hotplate. Begin recording the temperature and your observations of the ice/water every 0.5 minutes (in other words, every 30 seconds) in the observations table. Your second observation will be at 0.5 minute, your third will be at 1.0 minute, your fourth will be at 1.5 minutes, etc. 6. When the water begins to boil, record the temperature of the water for 3 more minutes, then turn off the hot plate and clean up your bench. Haugen/Yost Name: __________________________ Date: ______________ Period: _____ Marks ___________ OBSERVATIONS/DATA: 1. Data Table for Heating Curve of Water Time (minutes) Temperature (C) 0.0 0.5 1.0 1.5 Description of Water (Is it ice, liquid water, or steam, or a combination of these phases?) Haugen/Yost Name: __________________________ Date: ______________ Period: _____ Marks ___________ 2. Graph for Heating Curve of Water – include at the back of your lab report. DISCUSSION: A. Compare the temperature data to the graph on page 52 of your book. Based on this comparison and what you saw happening during the experiment fill in the numbered blanks. 12 TEMPERATURE (C) 13 6 10 11 7 9 4 1 8 2 5 3 TIME (Heat Energy) Word Bank: Condensing Freezing Melting Cooling Gas Solid Cooling Heating Liquid Cooling Heating Evaporating Heating Haugen/Yost 1. 2. 3. 4. 5. 6. 7. Name: __________________________ Date: ______________ Period: _____ Marks ___________ 8. 9. 10. 11. 12. 13. B. At which parts of the graph does the addition of heat energy cause an increase in temperature? C. At which parts of the graph is there no temperature change despite an addition of energy? What is happening to the water at points where there is no temperature change? D. Is the change from ice to water a physical or a chemical change? Explain.