dr._mala_-_lab_exercise_1_

Lab Exercise 1 - Scientific Observation & Measurement
The Scientific Method & Metric System
Introduction:
Scientific Method:
Science is a body of knowledge which will give you information about nature. The scientific method is used to gather that knowledge and to interpret it. It is also used to test the predictions of possible answers to questions about nature in ways that we can duplicate or verify. Answers supported by test results are added to the body of scientific knowledge and contribute to the concepts present in science.
Although these concepts are as up to date as possible, they are open to further questions and modifications.
Metric Conversion:
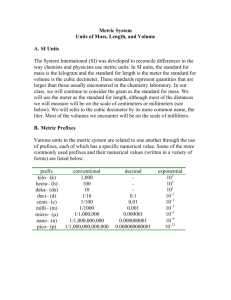
One requirement of the scientific method is that results must be repeatable. In many cases the numerical results are given as measurements of scientific experimentation. The most scientifically and internationally recognized system of measurement is the metric system . Units in the metric system are all based on multiples of ten . It is similar to the United States monetary system – ten pennies in a dime, ten dimes in a dollar. The reference units for the metric system are the meter for length, the liter for volume, the gram for mass and degree Celsius for temperature. It is relatively easy to convert from one measurement to another either by multiplying or dividing by 10 or a multiple of 10. Conversions from one unit to another can also be done quickly by shifting the decimal points.
Objectives:
Upon completion of this lab exercise the student will be able to
1.
2.
3.
4.
5.
List the five steps which constitute the scientific method.
Using the scientific method, create an experiment.
Demonstrate the ability to apply the scientific method by solving one hypothetical problem.
Use the units of measurements in the metric system for length, weight, volume and temperature.
Define all bold print and underlined words in the reading.
Steps Involved in Scientific Method (Figure 1.1):
Step 1:
Observation - The scientific method starts with careful observation. An investigator may make observations from nature or from the written words of other investigators.
Step 2:
Question - In the second step of the scientific method, one asks a question about these observations. The quality of this question will depend on how carefully the observations were made and analyzed.
Step 3:
Hypothesis - In this step, one would give a tentative solution to the question. We derive this hypothesis by inductive reasoning of a possible answer to the question.
Step 4:
Experiment - In this step one performs an experiment to test the hypothesis. In the experiment, the individuals or items under study are divided into two groups: an experimental group and a control group . In any test there are two kinds of variables: the independent variable , which is not affected by other items in or conditions of the experiment(s) and dependant variable , which can be affected by other items in or conditions of the experiment(s).
Step 5:
Conclusion - To make a conclusion is the last step in the scientific method. One uses the results of the experiment and pertinent data to evaluate the hypothesis. If the prediction is not supported, then the hypothesis is falsified. If the prediction is supported, the hypothesis is validated. One can never, however, completely accept or reject any hypothesis. All you can do is state whether it is correct or incorrect. There could be some unforeseen factors causing the result. For this reason, the results of the experiments must be repeatable by the original investigator and others.
Theories and Principles :
When exhaustive experiments and other data consistently support a hypothesis, it is accepted as a theory . A theory that stands the test of time may be elevated to the status of a principle . Theories and principles, however, can be modified or even discarded in the light of new knowledge. Biology, like life itself, is not static but is constantly changing.
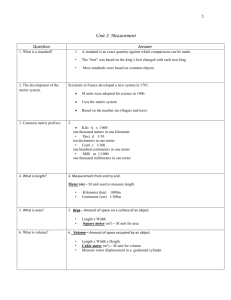
Measurement
Length:
Length is the measure of a real or imaginary line extending from one point to another. For measuring length and width we use the ruler , graduated in metric or English units. The standard unit is the meter ,
and comparing it to the English system, the meter is equal to approximately 39 inches. The meter is divided into 10 equal portions called decimeters (dm). Each decimeter is further divided into 10 equal parts called centimeter (cm). Each centimeter is again divided into 10 parts called millimeters (mm).
Below is a summary of the system with an analogy to the US monetary system.
1 meter is like 1 dollar
10 decimeters is like 10 dimes
100 centimeters is like 100 pennies
1000 millimeters
Kilometer:
1000 m = 1 kilometer (km)
1 kilometer is approximately equivalent to 5/8 of a mile.
Microscopic Measurement:
For the purpose of microscopic measurement, some units have been added. The most commonly used unit is the micrometer (µm) which is 1/1000 of a millimeter or 1/ 1,000,000 of a meter .
Note that the names of metric length units, other than the meter, contain prefixes to indicate how the unit relates to a meter. It is important to learn these prefixes and their meanings, because one will encounter them over and over again through out the study of the metric system. The prefixes are listed below.
Volume:
It is the space occupied by an object or a fluid. In the metric system, the standard unit of volume is the liter (l), but milliliters (ml) are the common units used for small volumes. 1cc (cubic centimeter) equals 1ml (milliliter). Graduated cylinders , beakers , conical flasks , and pipettes are used to measure fluids.
Mass:
Mass is the quantity of matter in an object. Mass is not the same as weight, because weight is dependent on the force of gravity acting on the object. The mass of a given object is the same on earth as on the moon, but its weight is much less on the moon because the moon’s force of gravity is less than the earth’s. You will use a triple-beam balance to measure the mass of an object. The standard metric unit of weight is the gram (g) . For measuring larger weights, the unit kilogram (kg) is usually used. A kilogram is equivalent to 1000 grams. A number of units are used to measure very small weights such as the milligram (mg).
Temperature:
The degree of hot or cold of an object is termed temperature . Thermometers are instruments used to measure temperature. Scientists use the degree Celsius ( o C) as the base unit for temperature measurements while most nonscientists use the degree Fahrenheit ( o F) . You can convert from one system to the other by using these formulas:
Celsius to Fahrenheit:
o
C
→ o
F o F = (C * 9/5) + 32
Fahrenheit to Celsius:
o F → o
C o C = (F - 32) * 5/9
In the Celsius system, water freezes at 0 o
C and boils at 100 c
. Room temperature falls between
20 – 25 o
C.
Metric Conversion
Length
Millimeter is abbreviated as mm ………………. (The Smallest)
10 millimeter (mm) = 1 centimeter (cm)
10 centimeter cm = 1 decimeter (dm)
10 decimeter (dm) = 1 meter (m)
10 meters (m) = 1 decameter (dam)
10 decameter (dam) = 1 hectometer (hm)
10 hectometer (hm) = 1 kilometer (km) ………….. (The Largest)
The value of each small division is 1 mm.
Volume
Milliliter is abbreviated as ml.
10 milliliter (ml) = 1 centiliter (cl)
10 centiliter (cl) = 1 deciliter (dl)
10 deciliter (dl) = 1 liter (l)
10 liter (l) = 1 decaliter (dal)
10 decaliter (dal) = 1 hectoliter (hl)
10 hectoliter (hl) = 1 kiloliter (kl)
Weight
Milligram is abbreviated as mg.
10 milligram (mg) = 1 centigram (cg)
10 centigram (cg) = 1 decigram (dg)
10 decigram (dg) = 1 gram (g)
10 gram (g) = 1 decagram (dag)
10 decagram (dag) = 1 hectogram (hg)
10 hectogram (hg) = 1 kilogram (kg)
1 meter = 39.37 inches
1 gram = 0.04 oz (ounce)
1 cubic centimeter (cc) = 1 ml
1 kg = 2.2 lb (pound)
1 inch = 2.54 cm
1 ounce = 29.57 ml
Conversion from Small to big, you will Divide or move the decimal point to the left .
Conversion from Big to small, you will Multiply or move decimal points to the right
.
Measuring Instruments (Figures 1.3 through 1.9):
Figure 1.2
-
Ruler Graduated in Inches and Centimeters
9
Figure 1.3
-
Triple Beam Balance
Figure 1.4
-
Graduated Figure 1.5
–
Beaker Figure 1.6
-
Conical Flask
Cylinder
Figure 1.7
-
Plastic Dropper
Figure 1.8 Thermometer
Prefix hydro hemo hyper hypo intra mono morph micro macro multi proto photo a or an meso endo aero anti amphi aqua arthro auto bi bio carne chloro chromo cide coel cyto di ecto (exo) endo epi foli gastro geo herba hetero homo
Common prefixes, Suffixes, and Root Words
Meaning not or non middle
Suffix /
( Pre/suffix ) asis, asia algia inner, inside blast needing oxygen or air clast against cyst both, doubly water joint self two, twice, double life, living cene cephal derm emia fer flesh genesis gram ia, ism itis green color killer, kill, killing hollow cell two, double itis lateral logy lys outer, external internal above leaf meter osis phase pod stomach phage pertaining to earth phobia plants different philia alike, similar water blood plasm phyll phyte saccharide above below within, inside one, single form small sis som spore stasis sub large many first synthesis tachy tropic troph light therm
Meaning condition of pain bud, sproud break down, broken bladder, pouch new. recent head skin condition of blood to bear origin, beginning writing, record condition of inflammation disease, inflammation side study of break down measurement a condition, disease stage foot to eat dislike, fear like form leaf plant sugar condition or state body seed stationary condition lesser, below to make quick, swift change, influence eat, consume heat
poly many vore swallow, devour sub lesser, below zoo, zoa animal
The main reason students find it difficult to understand Biology is because of all the hard to write, spell and read words.
Actually, scientific terminology is a hodgepodge of little words that are linked together to have different meanings. If you learn the meanings of the little words, you'll find scientific terminology much easier to understand.
Lab Activity 1 - Scientific Observation & Measurement - The Scientific Method & Metric
System
Required Materials:
2 Leaves from different types of plants
Ruler (metric)
5 Penny coins
Triple beam balance
2 different size Measuring cylinders (1small and 1 medium size)
Water bottle
2 different size Test Tubes (1 small size, and 1 medium size)
50-ml beaker (2)
500-ml beakers (2)
Hot plate
Tongs
2 Thermometers
_____________________________________________
Assignment 1
Metric System - Length
You must have observed that all plant leaves do not look alike. For example leaves of oak trees are different from maple. Similarly one leaf of a plant differs in size from the other.
Measure the length and width of the given leaves using a ruler and place your answers in the
Lab Report portion of this exercise (assignment 1).
Figure 1.9 Measuring Using the Metric System
Assignment 2
Metric System - Weight
Using the triple beam balance (Figure 1.12) , determine the weight of coins (pennies) and place your answers in the Lab Report portion of this exercise (assignment 2).
Figure 1.10 Triple Beam Balance
Assignment 3
Metric System - Volume
Take a test tube and fill with water. Measure the amount of water in the test tube by pouring that water into a graduated cylinder and place your answers in the Lab Report portion of this exercise (assignment 3).
Figure 1.11 Pouring & measuring water
Assignment 4
Metric System - Temperature
Take 50ml cold water from the tap and record its temperature. Allow the end bulb of the centigrade thermometer to remain in the water for 1 minute. Similarly take 50ml hot water from the tap and record the temperature. Place your answers in the Lab Report portion of this exercise (assignment 4)
Figure 1.12a Cold Water Figure 1.12b Hot Water
Lab Report – 1 Scientific Observation & Measurement
The Scientific Method & Metric System
Name: ________________________________
Date: _______________
Class Index: ___________
Instructor: ____________________________
Before you begin filling out this lab report you must read Exercise 1 - Scientific Observation &
Measurement in your lab manual. Complete Assignments 1-7 below. You can use your Lab Manual results and Textbook to complete the information below.
Assignment 1
Leaf Measurement a) Length _____cm _____ in. b) Length _____cm _____ in.
Width _____cm _____ in.
Width _____cm _____ in.
Assignment 2
Weight of the Coins
Number of Coins Trial
Number
1 2 pennies
Weight in g Weight in mg
2 5 pennies
Assignment 3
Volume (using graduated cylinder) a) _____ml _____oz. of water is present in the given small test tube. b) _____ml _____oz. of water is present in the given medium test tube.
Assignment 4
Temperature
a) Temperature of cold water in 50ml beaker from faucet is__________ °C b) Temperature of hot water in 50 ml beaker from faucet is__________ °C
Assignment 5
Conversion a) 70
◦
F to C by applying the formula (F - 32) * 5/9 (Show your calculation). b) 50
◦
C to F by applying the formula (C * 9/5) + 32 (Show your calculation).
Assignment 6
The following scientific terms are important to the study of Biology. Use the following prefixes and suffixes to complete this assignment:
Prefixes: bio = life morph, -i, -o = shape or form endo-, ento-, intra = inner or within inter-, meta = between intra = within extra = outside macro = large micro = small histo = tissue mono-, uni- = one muti-, poly- = many path, -i, -o = disease
Suffixes: ology = study of pathy = disease
-ac, -al, -alis, -an, -ar, -ary = pertaining to
-elle = small
-osis = a condition, disease
-lysis = loosen or split apart
Biology
Unicellular
Pathology
Intercellular
__________________
____________
Microbiology
Morphology
____________
Assignment 7
Measuring mass
What is the mass of the based on the position of the pointers? _______________________grams
Assignment 8
Do the following conversions: a.
4000mg = ____________________g b.
2L = ____________________ml c.
150cm = ____________________mm d.
18km = ____________________ m e.
110g = ____________________ kg f.
250 m = ____________________ km
Assignment 9
Lab Record Notebook
Using the following steps of the scientific method as a tool, design any one scientific investigation to acquire knowledge. Carefully complete each step recording the required information below.
Basic Steps of the Scientific method include:
1. Observation
2. Questioning
3. Hypothesis
4. Experiment
5. Conclusion a) Observation (recognized scientific problem, event or phenomenon)
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________ b) Questioning
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________ c) Hypothesis
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
d) Experimentation
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________ e) Conclusion/Result
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________
_________________________________________________________________