GENERAL INFORMATION AND SYLLABUS
advertisement

GENERAL CHEMISTRY (01:160:162) - SUMMER 2010
LECTURER AND COURSE COORDINATOR: Dr. Nancy Marky
DOOLITTLE TRAILER, RM. 107; marky@rutchem.rutgers.edu
Welcome to Chemistry 162 at Rutgers University. We sincerely hope that you will find this course to
be a valuable part of your professional training, as well as an enjoyable experience. All the members of the
instructional staff of the course are committed to making General Chemistry as profitable as possible for you.
Your success in the course will also be our success - but we will have to do it together.
Please read the following hand out carefully, as it provides information about the organization of
Chemistry 162, the course policies and the procedures we will follow in testing and assigning grades.
REQUIRED MATERIALS
1) GENERAL CHEMISTRY FOURTH EDITION by Hill, Petrucci, McCreary and Perry. (Prentice
Hall).
2) SELECTED SOLUTIONS MANUAL by C. Alton Hassell.
3) A Scientific calculator (logarithms, exponentials, powers, roots, etc.). Calculators with memory
and graphing capabilities are absolutely forbidden during quizzes and exams and will be taken
away.
LECTURES (4 per week)
(M-TH: 9:00-10:40)
You must attend all lectures. Any changes in the course format, exams,
etc., will be announced at the start of lectures and you will be held
responsible to know these changes. BE ON TIME. Attendance is of
utmost importance since lectures will emphasize and clarify important
concepts. You should also attend recitations. Regular attendance will
be one of the factors in deciding your course grade.
RECITATIONS (3 per week)
You will be assigned to one of the recitation sections on the first day of
(M-W: 8:00-8:50 or 10:50-11:40) classes. Change of section is not allowed. Please be prepared, and do
not hesitate, to ask questions during recitations in order to utilize them
to their fullest potential. The questions need not be restricted to
assigned problems. Anything not clear in the textbook or lectures
should be discussed during recitations. There will be 4 quizzes during
recitations accounting for 100 points. NO MAKE-UP QUIZZES
WILL BE GIVEN AND AN ABSENCE WILL COUNT AS ZERO
UNLESS ON VERIFIABLE MEDICAL GROUNDS. Credit will
then be prorated on the basis of other 3 quizzes. Attendance in
recitation will also account for 25 points as per the following criterion.
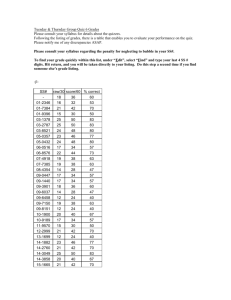
No. absent (excused or unexcused)
162 Summer Session I
1
Points
0-1
25
2
20
3
10
4
0
Gen. Info. & Syllabus
EXAMINATIONS
EXAMINATION FORMAT
AND GRADING
There will be no make-up examination. An absence from
examination for other than verifiable medical reasons will count as
a zero score in the missed exam. If you miss the examination on
medical grounds, see me as soon as possible. Exams will cover all
material assigned in the syllabus whether it is discussed in class or not.
You must have an ID, with your photograph, at the time of
examinations. Please arrive 15 minutes before the start of each exam.
No extra time will be allowed for late coming students.
All examinations will consist of multiple choice problems. All
necessary constants, periodic table and some formulae will be provided.
Examples from lecture notes and sample exam questions are invaluable
for good performance.
A tentative curve for grades will be made for each examination and
recitation. But the course grade will be based on the overall
performance in examinations, and recitation (total numerical score)
based on following credit.
Exam I
Exam II
Recitation quizzes
Attendance
Final Exam
100 pts.
100 pts.
100 pts.
25 pts.
200 pts.
The course grade will be solely based on your total point score. A single
point can make a difference. Further final exam and recitation quiz
scores will be given primary consideration for promotion of students
with borderline grades.
ACADEMIC INTEGRITY
University policies on academic integrity will be strictly enforced. Any
involvement with cheating, the fabrication or invention of information
used in an academic exercise, facilitating academic dishonesty of others
will result in serious consequences ranging from reprimand to expulsion.
EXTRA HELP
If you find that lectures, careful study, and recitations still leave you
with difficulties in understanding the course material, seek help early.
Make an appointment to see your recitation instructor or me. Extra help
is also available at MLSC (Busch) and Learning Resource Centers.
Contact them for details.
CHAIN OF COMMAND
In general, routine questions regarding course material, home-work
problems, quizzes, exam scores, absences, etc. should be directed first to
your recitation instructor. Only for further information, or if the above
procedure fails to resolve a particular problem, should you contact your
course lecturer.
162 Summer Session I
2
Gen. Info. & Syllabus
MATH SHEET
The following type of algebraic equations are often encountered while solving problems in Chemistry 162.
You should be able to solve these for X to succeed in the course. The answers are given in the parenthesis.
5. 56 10 3 X
0. 0110
1.
5. 56 10 3 X + 2.78
2.
[4.50/(4.50 + 1.21 X)] = 0.230 (12.4)
3.
3. 4
0. 42
3. 4 ( 27 / X )
(5.8)
4.
-4.54 = -2.23 + 2.48 ln (3.45/2.12 X)
(4.13)
5.
876 = -2.48 ln X
(4.21 10-154)
6.
X( 400 298)
= -(2.303)(8.31) log (4/8)
( 400 )( 298)
(6.73 103)
7.
X3/(2.0 - X)3 = 6.0
(1.3)
8.
X3
( 4 3X )( 7 2 X )
2
(5.56)
1.1 1013 (large # )
(1.33)
{X = (4/3 - 3.83 x 10-15}
9.
X
3
( 4 3X )( 7 2 X )
2
1. 4 10 14 (small # )
(1.4 10-4)
10.
0.259 = 31.4(1/24.5)X
(1.50)
11.
(2X)2(3X)3 = 1.1 10-18
(1.0 10-4)
12.
3.00 = 2.85 + log(1.8/X)
(1.3)
13.
3.7283 = (0.02568/2)ln X
(1.2 10126)
14.
0.20 = 0.158 - (0.02568/2)ln(X2/0.22)
(0.091)
15.
0.659 = 2.30X
(-0.5)
16.
2.303 log(X/1.4 10-2) = -4.31
(1.9 10-4)
17.
log(1.3 10-3) = log(5 1010) - (X/5.74 103)
(7.8 104)
162 Summer Session I
3
Gen. Info. & Syllabus
GENERAL CHEMISTRY 162 – SUMMER 2010 SYLLABUS
LEC #
DATE/DAY
READING
HOME-WORK PROBLEMS
1
6/1, T
11.1 - 11.4
MATH SHEET
11: 5,17,21,25,27,31,37,39,73
2
6/2, W
11.4 - 11.8
11: 10,43,49,53,56,57,83
3
6/3, Th
11.9 - 11.10/REVIEW
11:63,65,67,69,90
4
6/7, M
12.1 - 12.4
QUIZ 1 11.1 - 11.8
12: 27,29,33,35,39,41,43,45,81
5
6/8, T
12.5 - 12.7
12: 51,53,55,57,63,88,93
6
6/9, W
12.8 - 12.10
12: 65,69,71,75,79,91,98
QUIZ 2 11.9 - 12.4
7
6/10, Th
13.1 - 13.4/REVIEW
13: 23,25,27,29,31,35,89
8
6/14, M
LECTURE CANCELLED
EXAM I; 11.1 - 12.10
9
6/15, T
13.5 - 13.7
13: 37,41,43,47,55,79,90
10
6/16, W
13.8-13.11
13: 57,61,65,85
11
6/17, Th
14.1 - 14.3/REVIEW
14: 19,25,27,31,37,79,82
12
6/21, M
14.4 - 14.5
14: 43,47,49,51,53,57,59,63,69,85
162 Summer Session I
4
Gen. Info. & Syllabus
GENERAL CHEMISTRY 162 – SUMMER 2010 SYLLABUS
LEC #
DATE/DAY
READING
HOME-WORK PROBLEMS
13
6/22, T
15.1 - 15.3
15:21,23,25,31,35,37,39,41
14
6/23, W
15.4 -15.6
QUIZ 3 13.1 - 14.5
15: 47,48,51,57,59,63,67,69
15
6/24, Th
15.7 - 15.11/REVIEW
15:71,75,77,79,81,83,85,103
15: 89,93,97,99,100,101
16
6/28, M
LECTURE CANCELLED
EXAM II; 13.1 - 15.3
17
6/29, T
16.1 - 16.4
16: 19,23,27,35,39,41,45,47,49,53
18
6/30, W
16.5 - 16.6; 17.1 - 17.2
16: 63,67,69,71,73,77,83
QUIZ 4
15.4 -15.9
19
7/1, Th
17.3 -17.7 /REVIEW
17: 19,23,29,33,37,39,41
17: 45,51,55,61,63,75,87,94
20
7/6, T
18.1 - 18.5
18: 25,27,29,31,33,35,37,39,43,47,51,53
21
7/7, W
18.6; 18.9 - 18.10/ REVIEW
18: 57,59,61,63,75,79,81
22
7/8, Th
FINAL EXAM: 11.1 - 18.10
162 Summer Session I
5
Gen. Info. & Syllabus
QUIZ SCHEDULE
Quiz 1
Quiz 2
Quiz 3
Quiz 4
DATE/DAY
June 7,
M
June 9,
W
June 23,
W
June 30,
W
MATERIAL COVERED
11.1 - 11.8
11.9 - 12.4
13.1 - 14.5
15.4 - 15.9
EXAM SCHEDULE
Exam I
Exam II
Final
162 Summer Session I
DATE/DAY
June 14,
M
June 28,
M
July 8,
Th
6
MATERIAL COVERED
11.1 - 12.10
13.1 - 15.3
11.1 - 18.10
Gen. Info. & Syllabus