Unit 6
advertisement

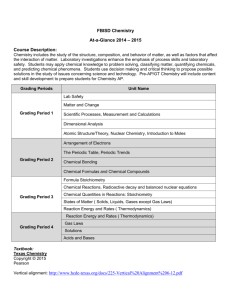
YOUNGSTOWN CITY SCHOOLS SCIENCE: CHEMISTRY UNIT #6 “STOICHIOMETRY” (4 WEEKS) 2013-2014 SYNOPSIS: In this unit students will investigate through lab work and practical problems the relationships among moles; Avogadro’s number; grams; molecules; atoms; and formula units. Students will use dimensional analysis to solve sample problems and tie together the different parts of Stoichiometry. A practical problem relating to the food industry will be presented to all students for a solution as the culminating activity. ENABLERS: isotopes, matter, mass, element, mole, macroscopic, formula mass, Avogadro’s number, density, stoichiometry, coefficients, molarities, percent yield, reagents STANDARDS VI. MOLES AND STOICHIOMETRY A. Matter can be quantified in a way that macroscopic properties such as mass can reflect the number of particles present B. Elemental samples are a mixture of several isotopes with different masses C. Atomic mass of an element is calculated given the mass and relative abundance of each isotope of the element as it exists in nature D. The mole is used to translate between the atomic and the macroscopic levels 1. The mole is used as a counting number, like a dozen. It is equal to the number of particles in exactly 12 grams of carbon (i.e. 12 atoms) 2. The mass of one mole of a substance is equal to its formula mass in grams E. The formula mass of a substance can be used in conjunction with Avogadro’s number and the density of a substance to convert between mass, moles, volume, and number of particles of a sample. F. A stoichiometric involves the conversion from the amount of one substance in a chemical reaction to the amount of another substance. G. The coefficients of the balanced equation indicate the ratios of the substances involved in the reaction in terms of both particles and moles. H. Once the number of moles of a substance is known, amounts can be changed to mass, volume of a gas, volume of solutions, and/or number of particles. I. Molarity is the measure of the concentration of a solution that can be used in stoichiometric calculations. J. the experimental yield can be compared to the theoretical yield to calculate percent yield LITERACY STANDARDS WHST. 7 Conduct short as well as more sustained research projects to answer a question (including self-generated question) or solve a problem; narrow or broaden the inquiry when appropriate; synthesize multiple sources on the subject, demonstrating understanding of the subject under investigation. TEACHER NOTES MOTIVATION 1. To introduce students to reactions, the teacher produces hydrogen from zinc, hydrochloric acid reaction; ignite the hydrogen. Have students write, balance, and classify the reaction. Teacher asks additional question such as - - how much hydrogen could we produce - - to set the stage for Stoichiometry unit. (VIJ) 2. Students set personal and academic goals 3. Preview the Authentic Assessment so students know what is expected by the end of the Unit TEACHER NOTES TEACHING-LEARNING 1. Teacher explains what Stoichiometry is and how it is through the concept of the mole, how it is calculated; Avogadro’s number; the relationship among moles, grams, molecules, atoms, and formula units. Students take notes for future reference, and use formulas in their practice problems. (VIA, VIB,VIC, VID) Holt Chemistry pages 100-10 and 224-248 and 302 – 327; All formulas and equations are in the chapters in the book 1 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 TEACHER NOTES TEACHING-LEARNING 2. Teacher gives students a question e.g., how much hydrogen will be produced from 10 grams of zinc? How much hydrochloric acid is needed to react with 10 grams of zinc? Students must connect the moles to the molecular weights so they can make calculations on what is needed to create a reaction (e.g., food industry, medicine, etc.) (VIF) 3. Teacher explains and demonstrates the role of dimensional analysis in Stoichiometry; explain the equations that will be needed to convert among the following: moles, grams, molecules, atoms, formula units, and Avogadro’s number. Students take notes and ask questions. Students work sample problems for each equation (Review problems as a class (VIE; VIF, VIG, VIH, VII) 4. Students conduct Lab: Mole Relationship in a Chemical Reaction; this will show students relationship between products and reactants. This will be related to industry or medicine to show how materials are put together. Lab attached on pages 4-5 (VIF) 5. Students conduct Lab: Water in a Hydrate (attached on pages 6-9) where students examine the amount of water in a compound and calculate grams of substance in a compound. This will show students the relationship between products and reactants, but with different equations and empirical formulas. (VIF) 6. Students complete Lab: Apple Pie; students mix ingredients in specific way focusing on measuring amounts; they are not ingredients that would normally be used in an Apple Pie, but it does taste like Apple Pie. (attached on page 10) (VIF) 7. Teacher discusses experimental yield, percent yield, theoretical yield and limiting reactants. Class has discussion and practice problems; students use a chemical equation and compare the reactants to products. (VIJ) (Empirical Formula Determination attached on pages 11-17) 8. Complete Hydrogen gas or CO2 gas to volume Lab (attached on pages 18-19) (NOTE: check for chemical supplies) (VIF, VIG, VIJ) 9. More sample problems, if needed for review prior to assessment. (VIA,VIB, VIC, VID, VIE; VIF, VIG, VIH, VII, VIJ). TEACHER NOTES TRADITIONAL ASSESSMENT 1. Unit Test: Multiple-Choice TEACHER NOTES TEACHER ASSESSMENT 1. Lab reports or practical reports, using rubrics for quality points. 2. Assignments/worksheets 3. 2- 4-point questions 2 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 TEACHER NOTES AUTHENTIC ASSESSMENT 1. Students evaluate their goals for the Unit. 2. Students complete the following: You are a candy maker developing a 20 oz. chocolate bar to put on the market. (a) Conduct research to determine what ingredients and how much of each ingredient you will need to produce 1000 pounds of chocolate to use in making your candy bars. (b) What other ingredients and how much of each will be needed to produce the candy bars. (c) If you want to make a 40% profit on your candy bars, how much will each candy bar cost? (d) Are there any other ways to produce a good tasting candy bar more cheaply? Discuss and share results with classmates whose research yielded different information than their own research.(WHST.7) 3 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 4 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 5 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 6 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 7 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 8 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 9 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 APPLE PIE LAB A vintage pie recipe made with saltine crackers and apple pie spice seasonings. Ingredients: pastry for 2-crust 9-inch pie 1 sleeve saltine crackers 2 cups water 2 cups granulated white sugar ½ cup brown sugar 2 teaspoons cream of tartar 2 tablespoons lemon juice 1 teaspoon grated lemon peel 1 tablespoon to 1/3 cup melted butter cinnamon Preparation: Line a 9-inch pie plate with rolled-out pastry. Break saltines coarsely into the pie shell. Combine water, sugar, and cream of tartar in a saucepan; bring to a boil. Simmer for 15 minutes. Add lemon juice and peel; cool. Pour the syrup over crackers. Dot with butter and sprinkle generously with cinnamon. Cover with top crust and flute edge. Cut slits into top to allow steam to escape. Bake in a 425° oven for 20 to 30 minutes, or until crust is golden brown and crisp. Serve warm, with ice cream, if desired. 10 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 11 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 12 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 13 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 14 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 15 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 16 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 17 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 18 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014 19 07/12/2013 YCS Science Chemistry Unit 6: Stoichiometry 2013-2014