Molar Mass of Butane Lab: Vapor Density Method
advertisement
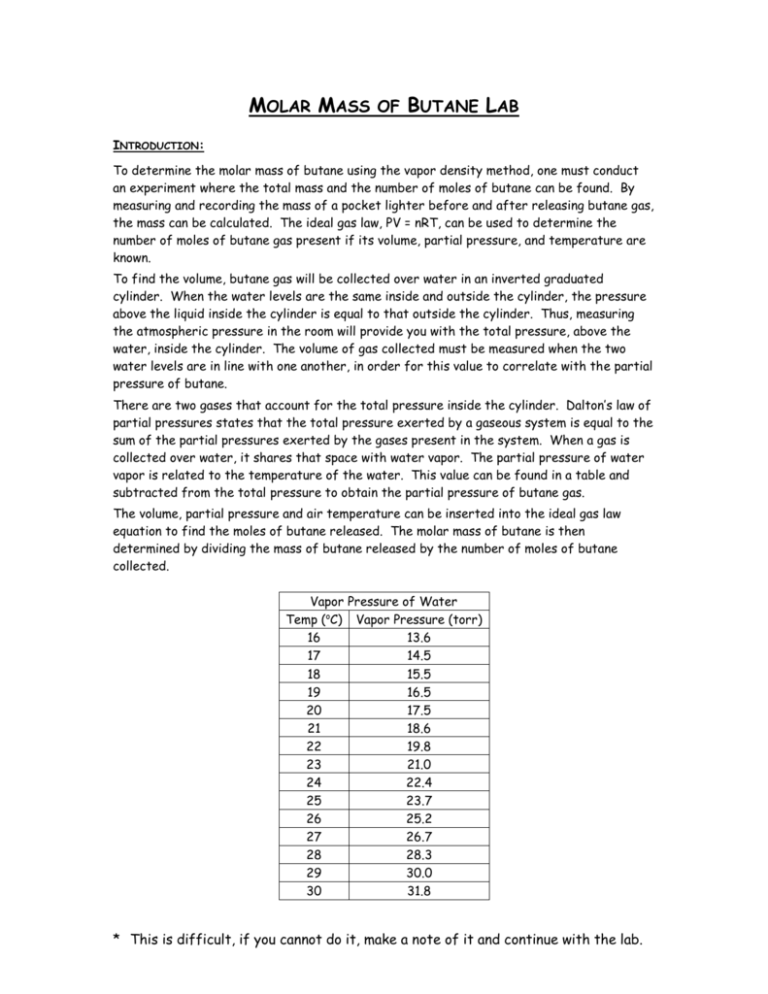
MOLAR MASS OF BUTANE LAB INTRODUCTION: To determine the molar mass of butane using the vapor density method, one must conduct an experiment where the total mass and the number of moles of butane can be found. By measuring and recording the mass of a pocket lighter before and after releasing butane gas, the mass can be calculated. The ideal gas law, PV = nRT, can be used to determine the number of moles of butane gas present if its volume, partial pressure, and temperature are known. To find the volume, butane gas will be collected over water in an inverted graduated cylinder. When the water levels are the same inside and outside the cylinder, the pressure above the liquid inside the cylinder is equal to that outside the cylinder. Thus, measuring the atmospheric pressure in the room will provide you with the total pressure, above the water, inside the cylinder. The volume of gas collected must be measured when the two water levels are in line with one another, in order for this value to correlate with the partial pressure of butane. There are two gases that account for the total pressure inside the cylinder. Dalton’s law of partial pressures states that the total pressure exerted by a gaseous system is equal to the sum of the partial pressures exerted by the gases present in the system. When a gas is collected over water, it shares that space with water vapor. The partial pressure of water vapor is related to the temperature of the water. This value can be found in a table and subtracted from the total pressure to obtain the partial pressure of butane gas. The volume, partial pressure and air temperature can be inserted into the ideal gas law equation to find the moles of butane released. The molar mass of butane is then determined by dividing the mass of butane released by the number of moles of butane collected. Vapor Pressure of Water Temp (oC) Vapor Pressure (torr) 16 13.6 17 14.5 18 15.5 19 16.5 20 17.5 21 18.6 22 19.8 23 21.0 24 22.4 25 23.7 26 25.2 27 26.7 28 28.3 29 30.0 30 31.8 * This is difficult, if you cannot do it, make a note of it and continue with the lab. PURPOSE: To determine the molar mass of butane. SAFETY: Do not ignite the pocket lighter or cause its flint to spark at any time during this experiment. MATERIALS: Butane Pocket Lighter 100mL Graduated Cylinder Thermometer Water Trough Balance Paper Towel PROCEDURE: 1. Clean and dry the butane lighter. Measure and record the mass of the lighter to the nearest 0.01g. Do not release any gas from the lighter after this measurement is taken unless you are instructed to do so. 2. Fill the water trough with more than enough water to completely submerge a 100mL graduated cylinder held horizontally. 3. Immerse the 100mL graduated cylinder in the water. Once you have removed all of the air bubbles inside the cylinder, invert the cylinder so that its mouth remains under the surface of the water. 4. Examine the cylinder to see if any air bubbles are present. Repeat steps 3 & 4 until you manage you invert the cylinder without allowing any air bubbles to get in. 5. Being careful to keep the mouth of the graduated cylinder under the water’s surface, raise the graduated cylinder up approximately an inch and secure it with a test tube clamp. 6. Immerse the lighter in the water trough and position it so the released gas bubbles will be trapped by the graduated cylinder. 7. Depress the lever that releases the butane gas. You will see gas bubble ascending up the graduated cylinder and collecting at the top. Make sure that all the gas bubbles enter the cylinder. Continue to hold down the lever until you have collected 90mL of gas. 8. Making sure that the mouth of the graduated cylinder remains under the surface of the water at all times, carefully raise and lower the cylinder until the water level inside the cylinder is the same as the water level outside the cylinder.* 9. Record the volume of gas in the graduated cylinder. 10. Dry off the lighter. 11. Measure and record the final mass of the lighter. 12. Measure and record the air temperature, temperature of the water and barometric pressure. 13. Repeat steps 1-11 two more times for a total of 3 trials. * This is difficult, if you cannot do it, make a note of it and continue with the lab. Name & Period: _________________________ MOLAR MASS OF BUTANE LAB PAGES TO BE TURNED IN PRE-LAB QUESTIONS: Answer the questions on a separate sheet of paper. 1. Why should there be no open flames in the lab during this experiment? 2. Why do we need to ensure that the inverted graduated cylinder contains only water (no air bubbles) before the butane gas is added? 3. For the purpose of this lab, explain why it is important that the butane is relatively insoluble in water. DATA: Trial 1 Initial Mass of Lighter Final Mass of Lighter Mass of Butane Volume of Gas Collected Air Temperature Atmospheric Pressure Water Temperature Vapor Pressure of Water Partial Pressure of Butane Moles of Butane Molar Mass of Butane Average Molar Mass of Butane Accepted Molar Mass of Butane Percent Error Trial 2 Trial 3 DATA ANALYSIS: Do your work on the same paper as your pre-lab questions. Show your calculations clearly with proper formulas and documentation. For parts 13, show your work for trial 1 only. 1. Determine the partial pressure of butane gas in the cylinder. 2. Determine the number of moles of butane gas collected in the cylinder. 3. Calculate the experimental molar mass of butane. 4. Calculate the average molar mass of butane. 5. The formula for butane is C4H10. Calculate the accepted molar mass of butane. 6. Determine the percent error. DISCUSSION: 1. Answer the questions on the same paper as your calculations. What are the measurements that must be taken to find the molar mass of a gas using the vapor density method? (Note: Calculations are not measurements.) 2. Explain why the water levels inside and outside the graduated cylinder must be the same before the volume of gas is measured and recorded. 3. Suppose a student failed to account for the partial pressure of water in the graduated cylinder. What effect would this have on the calculated value for the molar mass of butane? Justify your answer. 4. Calculate the dry volume (volume without water) of butane gas that was released from your lighter at the temperature and pressure in you lab on the day you conducted this experiment. Use the number of moles found in trial one. 5. Why is the dry volume of butane gas less than the wet volume measured in the lab? 6. Suppose that the lighter used by a student in this experiment was not completely dry before its final mass was measured. What effect would this have on the student’s calculated value for the molar mass of butane? Explain. * This is difficult, if you cannot do it, make a note of it and continue with the lab.









