BuffersAndKspFollowAlongNotes - ECHS Chemistry
advertisement

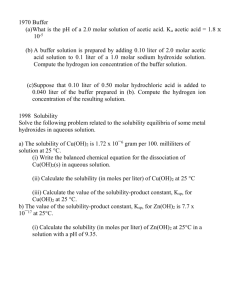
AP Chemistry: Chapter 15 Student Notes Objectives 15.1: 15.2: 15.3: 15.4: 15.5: 15.6: 15.7: The Common Ion Effect What are Buffers Buffer Capacity Titrations and pH Curves Acid-Base Indicators Solubility Equilbria and Ksp Ksp and Qualitative Analysis 15.1: The Common Ion Effect Sometimes equilibrium solutions can have more than one ion—common ions: NaF(s) __________ + ____________ This goes into the reaction: HF(aq) ↔ H+(aq) + (dissociation) F-(aq) Adding NaF does _________________ Example 1: What is the percent dissociation of: a. A 0.10-M solution of acetic acid: CH3COOH? b. A mixture that contains 0.10-M acetic acid and 0.10-M sodium acetate? Bergmann Page 1 3/7/2016 15.2: What are Buffers What is a buffer A buffer ____________ a change in pH A buffer must have Example 1 What is the pH of a solution where 50. mL of 0.50M NaC2H3O2 is mixed with 25mL of 0.25M HC2H3O2 A cool Equation: The Henderson-Hasselbach Equation [base] pH pKa log [acid ] Bergmann Page 2 3/7/2016 Example 2: Calculate the pH of the following solution: 25 mL of a 0.150M solution of hypochlorous acid (HOCl) and 32mL of a 0.45M potassium hypochlorite (KOCl) This can be simplified if you work in mmol throughout the problem Working in mmol only works if the solution is _______________________ Example 3: Calculate the pH when 25.0 mL of 0.50 M methylammonium nitrate is mixed with 75 mL of 0.30 M methylamine. Bergmann Page 3 3/7/2016 Adding Strong Acids and Bases to solutions: Equivalence Point: When the __________ of ____________ = the ____________ of ___________ How do you know why you have reached the equivalence point? 1. 2. Diagram for # 2 above Description of this experiment Bergmann Page 4 3/7/2016 Example 4:[Strong Acid + Salt of Weak Base] What is the pH when 20mL of 0.25M HCl is reacted with 20 mL of 0.35M sodium nitrite? Example 5: [Strong Acid + Buffer Solution] What is the pH when 15 mL of 0.20 M HNO3 is added to a buffer that contains 50.0 mL of a 0.25 M HCO2H and 0.30 M NaCO2H? Example 6: [Strong Base + Buffer Solution] What is the pH when 15 mL of 0.20 M NaOH is added to a buffer that contains 50.0 mL of a 0.25 M HCO2H and 0.30 M NaCO2H ? Bergmann Page 5 3/7/2016 Example 7: [Strong Base + Weak Base Buffer System]: What is the pH when 40 mL of 0.25M NaOH is added to a buffer that contains 100 mL of 0.40M Ethalmine (C2H5NH2) and 0.40M ethylammonium chloride (C2H5NH3Cl)? Example 8: [Strong Base + Weak Acid—ep] What is the pH when 40 mL of 0.25 M NaOH is mixed with 20 mL of 0.50M propanoic acid (HC3H5O2) Example 9: [Strong Base + Weak Acid—beyep] What is the pH when 42 mL of 0.25 M NaOH is mixed with 20 mL of 0.50M propanoic acid (HC3H5O2) Bergmann Page 6 3/7/2016 Example 10: [Strong Acid + Weak Base] What is the pH when 20 mL of 0.20 M methylamine(CH3NH2) is mixed with 10 mL of 0.20 M HNO3. Example 11: [Strong Acid + Weak Base] What is the pH when 20 mL of 0.20 M methylamine(CH3NH2) is mixed with 20 mL of 0.20 M HNO3. Example 11: [Strong Acid + Weak Base] What is the pH when 20 mL of 0.20 M methylamine(CH3NH2) is mixed with 24 mL of 0.20 M HNO3. Example 12: [Ratio Problem] The ratio of NH3 to NH4+ in a buffered solution is 3.2: What is the pH? Bergmann Page 7 3/7/2016 15.4: Titrations and pH Curves Strong Acid with Strong Base Strong Base Strong Acid Bergmann Page 8 3/7/2016 Weak Acid-Strong Base Strong Acid-Weak Acid with Strong Base Comparison Bergmann Page 9 3/7/2016 Ka and Titration Curves Weak Base with Strong Acid Bergmann Page 10 3/7/2016 A Special Point: when the pH = pKa Bergmann Page 11 3/7/2016 15.5: Acid-Base Indicators Equivalence Point is determined by 1. Colorometrically: 2. Using a pH meter: How do you pick an indicator—for colorometric analysis? 1. 2. Determine the pH at the equivalence point either from a calculation or using a pH meter Choose the appropriate indicator from the chart below: Indicator Gentian violet (Methyl violet) Low pH color Transition pH range High pH color yellow 0.0–2.0 blue-violet Leucomalachite green (first transition) yellow 0.0–2.0 green Leucomalachite green (second transition) green 11.6–14 colorless Thymol blue (first transition) red 1.2–2.8 yellow Thymol blue (second transition) yellow 8.0–9.6 blue Methyl yellow red 2.9–4.0 yellow Bromophenol blue yellow 3.0–4.6 purple Congo red blue-violet 3.0–5.0 red Bergmann Page 12 3/7/2016 Methyl orange red 3.1–4.4 orange Bromocresol green yellow 3.8–5.4 blue-green Methyl red red 4.4–6.2 yellow Methyl red / Bromocresol green red 4.5–5.2 green Azolitmin red 4.5–8.3 blue Bromocresol purple yellow 5.2–6.8 purple Bromothymol blue yellow 6.0–7.6 blue Phenol red yellow 6.8–8.4 purple Neutral red red 6.8–8.0 yellow Naphtholphthalein colorless to reddish 7.3–8.7 greenish to blue Cresol Red yellow 7.2–8.8 reddish-purple Phenolphthalein colorless 8.3–10.0 fuchsia Thymolphthalein colorless 9.3–10.5 blue Alizarine Yellow R yellow 10.2–12.0 red Bergmann Page 13 3/7/2016 Which indicator would you pick? Bergmann Page 14 3/7/2016 How do indicators work? All indicators are ____________________ Their “partners” have color and change when the equilibrium is shifted. HIn ↔ H+ + InBlue green Or some such derivation Each indicator has a Ka and the color change occurs at approximately the –log of the Ka. Example 1: The equivalence point of a titration is 4.3: Which of the following indicators would be a good choice? Explain your choice. Indicator Thymol Blue Eriochrome Black T Alizarin m-Nitrophenol Thymolphthalien Alizarin Yellow R Ka 2.3 x 10-2 5.4 x 10-5 6.6 x 10-6 8.3 x 10-8 2.5 x 10-9 4.3 x 10-11 15.6: Solubility Equilbria and Ksp Ksp: the study of the solubility’s of ____________ ionic ________________ Writing Solubility Product Expressions CaF2 Mg3(PO4)2 BaSO4 Ksp values are _______________ Bergmann Page 15 3/7/2016 Ksp Values Bromides Carbonates Hydroxides Iodides Oxalates Phosphates Bergmann PbBr2 AgBr BaCO3 CaCO3 CoCO3 CuCO3 FeCO3 PbCO3 MgCO3 MnCO3 NiCO3 Ag2CO3 ZnCO3 AgOH Al(OH)3 Ca(OH)2 Cr(OH)3 Co(OH)2 Cu(OH)2 Fe(OH)2 Fe(OH)3 Pb(OH)2 Mg(OH)2 Mn(OH)2 Ni(OH)2 Zn(OH)2 PbI2 AgI BaC2O4 CaC2O4 MgC2O4 AlP04 Ba3(P04)2 Ca3(P04)2 CrP04 Pb3(P04)2 Ag3P04 Zn3(P04)2 6.3 x 10-6 3.3 x 10-13 8.1 x 10-9 3.8 x 10-9 8.0 x 10-13 2.5 x 10-10 3.5 x 10-11 1.5 x 10-13 4.0 x 10-5 1.8 x 10-11 6.6 x 10-9 8.1 x 10-12 1.5 x 10-11 2.0 x 10-8 1.9 x 10-33 7.9 x 10-6 6.7 x 10-31 2.5 x 10-16 1.6 x 10-19 7.9 x 10-15 6.3 x 10-38 2.8 x 10-16 1.5 x 10-11 4.6 x 10-14 2.8 x 10-16 4.5 x 10-17 8.7 x 10-9 1.5 x 10-16 1.1 x 10-7 2.3 x 10-9 8.6 x 10-5 1.3 x 10-20 1.3 x 10-29 1.0 x 10-25 2.4 x 10-23 3.0 x 10-44 1.3 x 10-20 9.1 x 10-33 Chlorides Chromates Cyanides Fluorides Sulfates Sulfides Sulfites PbCl2 AgCl BaCrO4 CaCrO4 PbCrO4 Ag2CrO4 Ni(CN)2 AgCN Zn(CN)2 BaF2 CaF2 PbF2 MgF2 BaS04 CaS04 PbS04 Ag2S04 CaS CoS CuS FeS Fe2S3 PbS MnS NiS Ag2S ZnS BaS03 CaS03 Ag2S03 Page 16 1.7 x 10-5 1.8 x 10-10 2.0 x 10-10 7.1 x 10-4 1.8 x 10-14 9.0 x 10-12 3.0 x 10-23 1.2 x 10-16 8.0 x 10-12 1.7 x 10-6 3.9 x 10-11 3.7 x 10-8 6.4 x 10-9 1.1 x 10-10 2.4 x 10-5 1.8 x 10-8 1.7 x 10-5 8 x 10-6 5.9 x 10-21 7.9 x 10-37 4.9 x 10-18 1.4 x 10-88 3.2 x 10-28 5.1 x 10-15 3.0 x 10-21 1.0 x 10-49 2.0 x 10-25 8.0 x 10-7 1.3 x 10-8 1.5 x 10-14 3/7/2016 Example 1: The solubility of copper I bromide is 2.0 x 10-4M. What is the value of Ksp Example 2: What is the molar solubility of Silver Sulfide? Example 3: What is the molar solubility of Bismuth III Sulifde. The Ksp = 1.1 x 10-73 Example 4: What is the molar solubility of Iron III hydroxide Silly Hydroxides Bergmann Page 17 3/7/2016 Will a ppt form: Compare the Ksp to the Qsp Qsp> Ksp ________________ Qsp < Ksp ________________ Example 5: Will a ppt form 50.0 mL of 0.00025 M Na3PO4 is mixed with 50.0 mL of 0.0025 M BaCl2. Will a ppt form? Show all calculations to support your answer. Example 6: Competing ppt Sodium chloride is added to a 50 mL beaker that contains a mixture or 0.00015 M lead II nitrate and 0.00035 M silver nitrate. What ppt will form first. Show all work. Bergmann Page 18 3/7/2016 Example 7: Competing ppt and how much remains. At 25˚C the solubility product constant, Ksp, for strontium sulfate, SrSO4, is 7.610-7. The solubility product constant for strontium fluoride, SrF2, is 7.910-10. (a) What is the molar solubility of SrSO4 in pure water at 25˚C? (b) What is the molar solubility of SrF2 in pure water at 25˚C? (c) An aqueous solution of Sr(NO3)2 is added slowly to 1.0 litre of a well-stirred solution containing 0.020 mole F- and 0.10 mole SO42- at 25˚C. (You may assume that the added Sr(NO3)2 solution does not materially affect the total volume of the system.) 1. Which salt precipitates first? 2. What is the concentration of strontium ion, Sr2+, in the solution when the first precipitate begins to form? (d) As more Sr(NO3)2 is added to the mixture in (c) a second precipitate begins to form. At that stage, what percent of the anion of Bergmann Page 19 3/7/2016 AP Chemistry Chapter 15 Practice Exam 1. A buffer solution contains 0.40 mole of formic acid, HCOOH, and 0.60 mole of sodium formate, HCOONa, in 1.00 liter of solution. The ionization constant, Ka, of formic acid is 1.8x10-4. (a) Calculate the pH of this solution. (b) If 100. milliliters of this buffer solution is diluted to a volume of 1.00 liter with pure water, the pH does not change. Discuss why the pH remains constant on dilution. (c) A 5.00 milliliter sample of 1.00 molar HCl is added to 100. milliliters of the original buffer solution. Calculate the [H3O+] of the resulting solution. (d) A 800.-milliliter sample of 2.00-molar formic acid is mixed with 200. milliliters of 4.80-molar NaOH. Calculate the [H3O+] of the resulting solution. Bergmann Page 20 3/7/2016 2. In water, hydrazoic acid, HN3, is a weak acid that has an equilibrium constant, Ka, equal to 2.8x10-5 at 25ºC. A 0.300 liter sample of a 0.050 molar solution of the acid is prepared. (a) Write the expression for the equilibrium constant, Ka, for hydrazoic acid. (b) Calculate the pH of this solution at 25ºC. (c) To 0.150 liter of this solution, 0.80 gram of sodium azide, NaN3, is added. The salt dissolved completely. Calculate the pH of the resulting solution at 25ºC if the volume of the solution remains unchanged. (d) To the remaining 0.150 litre of the original solution, 0.075 liter of 0.100 molar NaOH solution is added. Calculate the [OH-] for the resulting solution at 25ºC. Bergmann Page 21 3/7/2016 3. The equations and constants for the dissociation of three different acids are given below. HCO3- <=> H+ + CO32Ka = 4.2 x 10-7 H2PO4- <=> H+ + HPO42Ka = 6.2 x 10-8 HSO4- <=> H+ + SO42Ka = 1.3 x 10-2 (a) From the systems above, identify the conjugate pair that is best for preparing a buffer with a pH of 7.2. Explain your choice. (b) Explain briefly how you would prepare the buffer solution described in (a) with the conjugate pair you have chosen. (c) If the concentrations of both the acid and the conjugate base you have chosen were doubled, how would the pH be affected? Explain how the capacity of the buffer is affected by this change in concentrations of acid and base. (d) Explain briefly how you could prepare the buffer solution in (a) if you had available the solid salt of the only one member of the conjugate pair and solution of a strong acid and a strong base. Bergmann Page 22 3/7/2016 4. The solubility of iron(II) hydroxide, Fe(OH)2, is 1.43x10-3-gram per litre at 25oC. (a) Write a balanced equation for the solubility equilibrium. (b) Write the expression for the solubility product constant, Ksp, and calculate its value. (c) Calculate the pH of a saturated solution of Fe(OH)2 at 25oC. (d) A 50.0-millilitre sample of 3.00x10-3 molar FeSO4 solution is added to 50.0millilitres of 4.00x10-6-molar NaOH solution. Does a precipitate of Fe(OH)2 form? Explain and show calculations to support your answer. Bergmann Page 23 3/7/2016 5. Solve the following problem related to the solubility equilibria of some metal hydroxides in aqueous solution. (a) The solubility of Cu(OH)2(s) is 1.72x10-6-gram per 100. milliliters of solution at 25°C. (i) Write the balanced chemical equation for the dissociation of Cu(OH)2(s) in aqueous solution. (ii) Calculate the solubility (in moles per liter) of Cu(OH)2 at 25oC. (iii) Calculate the value of the solubility-product constant, Ksp, for Cu(OH)2 at 25oC. (b) The value of the solubility-product constant, Ksp, for Zn(OH)2 is 7.7x10-17 at 25oC. (i) Calculate the solubility (in moles per liter) of Zn(OH)2 at 25oC in a solution with a pH of 9.35. (ii) At 25oC, 50.0-milliliters of 0.100-molar Zn(NO3)2 is mixed with 50.0-milliliters of 0.300-molar NaOH. Calculate the molar concentration of Zn2+(aq) in the resulting solution once equilibrium has been established. Assume that volumes are additive. Bergmann Page 24 3/7/2016