Singapore Quality Overall Summary for Chemical Drugs
advertisement
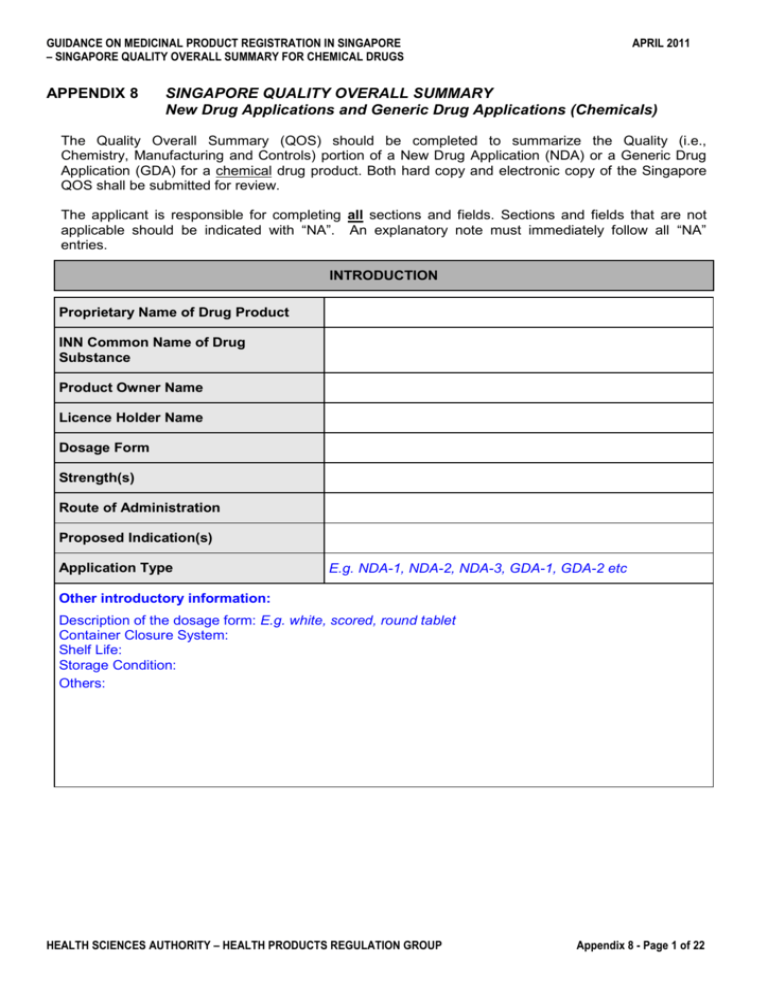
GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APPENDIX 8 APRIL 2011 SINGAPORE QUALITY OVERALL SUMMARY New Drug Applications and Generic Drug Applications (Chemicals) The Quality Overall Summary (QOS) should be completed to summarize the Quality (i.e., Chemistry, Manufacturing and Controls) portion of a New Drug Application (NDA) or a Generic Drug Application (GDA) for a chemical drug product. Both hard copy and electronic copy of the Singapore QOS shall be submitted for review. The applicant is responsible for completing all sections and fields. Sections and fields that are not applicable should be indicated with “NA”. An explanatory note must immediately follow all “NA” entries. INTRODUCTION Proprietary Name of Drug Product INN Common Name of Drug Substance Product Owner Name Licence Holder Name Dosage Form Strength(s) Route of Administration Proposed Indication(s) Application Type E.g. NDA-1, NDA-2, NDA-3, GDA-1, GDA-2 etc Other introductory information: Description of the dosage form: E.g. white, scored, round tablet Container Closure System: Shelf Life: Storage Condition: Others: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 1 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S DRUG SUBSTANCE S 1 GENERAL INFORMATION Check appropriate box. DMF (open) part is attached. DMF (open and restricted) and Letter of Access to be submitted by DDMMYYYY (within one month of PRISM submission), OR Letter of Access to the DMF filed with HSA (015:________) is provided. * CEP (Certificate of Suitability from EDQM) for Drug Substance is attached. CEP Number: CEP (Certificate of Suitability from EDQM) for Raw materials and Excipients is attached. Drug Substance meets the current USP/PhEur/BP/JP (delete as appropriate) requirements. Drug Substance meets other pharmacopoeia standards. Analytical methods and appropriate analytical method validation data are included in the dossier. Drug Substance meets in-house specifications. Analytical methods and appropriate analytical method validation data are included in the dossier. * If CEP is provided and Ph.Eur standard is claimed for drug substance, please fill in S1, S2.1, S4.1, S4.4, S6 # and S7# If CEP is provided and other standards are claimed for drug substance, please fill in S1, S2.1, S4.1 to S4.5, S6 # and S7# (#To be provided if re-test period/shelf life is not stated on CEP) S 1.1 Nomenclature Hard Copy Location/Pages: E-Copy Location/File Name: Chemical Name: Other names: (e.g. INN, BAN, USAN, common name) Company or laboratory code: Chemical Abstracts Service (CAS) registry number: S 1.2 Structure Hard Copy Location/Pages: E-Copy Location/File Name: Structural formula (including stereochemistry): [insert structure] Molecular formula: Molecular Mass: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 2 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S 1.3 General Properties Hard Copy Location/Pages: E-Copy Location/File Name: Physical description (e.g., appearance, colour, physical state): Physical form (e.g., polymorphic form, solvate, hydrate): Solubilities (e.g., in common solvents, aqueous/non-aqueous solubility profile): pH and pKa values: Other (e.g., partition coefficients, melting or boiling points, optical rotation, refractive index (for a liquid), hygroscopicity, UV absorption maxima and molar absorptivity): S 2 MANUFACTURE S 2.1 Manufacturer(s) Name, address, and activity of each manufacturer, including contractors, and each proposed production site or facility involved in manufacture and testing: Activity Name and Address *GMP Compliance (Please indicate Approving Agency) Site of Manufacture Site of Batch Release * For information only. S 2.2 Description of Manufacturing Process and Process Controls Hard Copy Location/Pages: E-Copy Location/File Name: Flow diagram of the synthetic process(es): [insert diagram] S 2.3 Control of Materials Hard Copy Location/Pages: E-Copy Location/File Name: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 3 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S 2.4 Controls of Critical Steps and Intermediates Hard Copy Location/Pages: E-Copy Location/File Name: S 2.5 Process Validation and/or Evaluation Hard Copy Location/Pages: E-Copy Location/File Name: S 2.6 Manufacturing Process Development Hard Copy Location/Pages: E-Copy Location/File Name: S 3 CHARACTERISATION S 3.1 Elucidation of Structure and other Characteristics Hard Copy Location/Pages: E-Copy Location/File Name: S 3.2 Impurities Summary of potential and actual impurities arising from the synthesis, manufacture and/or degradation: Chemical Name/Laboratory Code Origin/Type of Impurity Structure [insert structure] Process-related impurities (e.g., residual solvents): Compound Name HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Step in Process Appendix 8 - Page 4 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Process-related impurities (e.g., residual solvents): Compound Name Step in Process S 4 CONTROL OF THE DRUG SUBSTANCE S 4.1 Drug Product Manufacturer’s Specification Standard Claimed for the Drug Substance (e.g., USP, BP, etc.): Test Method (e.g., HPLC) Source (e.g., USP, inhouse) HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Release Specification Shelf Life Specification (if applicable) Appendix 8 - Page 5 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S 4.1 Drug Substance Manufacturer’s Specification (if different from above) Standard Claimed for the Drug Substance (e.g., USP, BP, etc.): Test Method (e.g., HPLC) Source (e.g., USP, inhouse) HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Release Specification Shelf Life Specification (if applicable) Appendix 8 - Page 6 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S 4.2 Analytical Procedures (Drug Product Manufacturer) S 4.3 Validation of Analytical Procedures (Drug Product Manufacturer) For each test, please indicate “yes” or “no” as appropriate System Suitability Or Others (Please specify) Robustness Limit of Quantitation Limit of Detection Precision - Repeatability - Intermediate Precision - Reproducibility Accuracy Range Linearity Selectivity Method Description Test Name (as per S4.1) S 4.2 Analytical Procedures (Drug Substance Manufacturer, if different from above) S 4.3 Validation of Analytical Procedures (Drug Substance Manufacturer, if different from above) For each test, please indicate “yes” or “no” as appropriate System Suitability Or Others (Please specify) Robustness Limit of Quantitation HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Limit of Detection Precision - Repeatability - Intermediate Precision - Reproducibility Accuracy Range Linearity Selectivity Method Description Test Name (as per S4.1) Appendix 8 - Page 7 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 S 4.4 Batch Analyses Typical production batch size: Batch Number Batch Size Batch Type (pilot/production) Date of Production Site of Production S 4.5 Justification of Specification Hard Copy Location/Pages: E-Copy Location/File Name: Test Justification of Specifications S 5 REFERENCE STANDARDS OR MATERIALS Hard Copy Location/Pages: E-Copy Location/File Name: Drug Substance Batch Number Source (e.g., USP, in-house) Batch Number Source (e.g., USP, in-house) Primary Reference Standard Working Standard Impurities Primary Reference Standard Working Standard S 6 CONTAINER CLOSURE SYSTEM HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 8 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Description of the container closure system(s) for the storage of the drug substance: S 7 STABILITY S 7.1 Stability Summary and Conclusions Summary and discussion of all stability study results: Hard Copy Location/Pages: E-Copy Location/File Name: Proposed Production Batch Size (kg): Batch Number Batch Size Storage Conditions (°C, % RH, light) Date of Manufacture Site of Manufacture Batch Number Container Closure System Completed Test Intervals E.g. 0, 3, 6, 9, 12, 18, 24, 36 months Proposed storage conditions and re-test period (or shelf life, as appropriate): Container Closure System Storage Conditions Re-test Period Shelf Life If applicable If applicable S 7.2 Post-approval Stability Protocol and Stability Commitment Hard Copy Location/Pages: E-Copy Location/File Name: Stability protocol for commitment batches (if applicable): Protocol Parameter HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Description Appendix 8 - Page 9 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Stability protocol for commitment batches (if applicable): Protocol Parameter Description Number of batches and batch sizes Tests and acceptance criteria Container closure system(s) Testing frequency Storage conditions (and tolerances) of samples Other S 7.3 Stability Data Hard Copy Location/Pages: E-Copy Location/File Name: P DRUG PRODUCT P 1 DESCRIPTION AND COMPOSITION OF THE DRUG PRODUCT (1) Description of the Dosage Form: Presence of Score Line: Yes / No (delete as appropriate) (2) Composition, i.e., list of all components of the dosage form, and their amounts on a per unit basis (including overages, if any): Strength (Label claim): Components Quality Standard Quantity per unit % Function Total HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 10 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 (3) Composition, i.e., qualitative list of all components of proprietary materials (e.g., capsule shells, colouring blends, imprinting inks, etc.): Proprietary Material Qualitative Composition Quantitative Composition (4) Description of accompanying reconstitution diluent(s), if applicable: P 2 PHARMACEUTICAL DEVELOPMENT P 2.1 Components of the Drug Product Hard Copy Location/Pages: E-Copy Location/File Name: P 2.2 Drug Product P 2.2.1 Formulation Development Hard Copy Location/Pages: E-Copy Location/File Name: P 2.2.2 Overages Hard Copy Location/Pages: E-Copy Location/File Name: P 2.2.3 Physicochemical and Biological Properties Hard Copy Location/Pages: E-Copy Location/File Name: P 2.3 Manufacturing Process Development Discussion of the development of the manufacturing process of the drug product (e.g., optimization of the process, selection of the method of sterilization, etc.): Hard Copy Location/Pages: E-Copy Location/File Name: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 11 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 P 2.4 Container Closure System Discussion of the suitability of the container closure system (described in P 7) used for the storage, transportation (shipping), and use of the drug product (e.g., physicochemical tests, biological reactivity tests, leaching, etc.): Hard Copy Location/Pages: E-Copy Location/File Name: P 2.5 Microbiological Attributes Discussion of microbiological attributes of the dosage form (e.g., preservative effectiveness studies): Hard Copy Location/Pages: E-Copy Location/File Name: P 2.6 Compatibility Discussion of the compatibility of the drug product with reconstitution diluent(s) or dosage devices (e.g., precipitation of drug substance in solution, sorption on injection vessels, etc.): Hard Copy Location/Pages: E-Copy Location/File Name: P 3 MANUFACTURE P 3.1 Manufacturer(s) Name, address, and activity of each manufacturer, including contractors, and each proposed production site or facility involved in manufacture and testing of product intended for Singapore: Activity Name and Address Site of Fabrication, Manufacturing Site of Primary Packaging Site of Secondary Packaging Site of Batch Release P 3.2 Batch Formula List of all components of the dosage form to be used in the manufacturing process, and their amounts on a per batch basis (including overages, if any): HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 12 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Strength (Label claim): Batch Size (Number of dosage units): Please provide batch formula for all proposed production batch sizes. If a batch range is proposed, the minimum and maximum batch formula should be provided. Component Quality Standard (or Grade) Quantity per batch Total P 3.3 Description of Manufacturing Process and Process Controls Hard Copy Location/Pages: E-Copy Location/File Name: Flow diagram of the manufacturing process(es): [insert diagram] P 3.4 Controls of Critical Steps and Intermediates Hard Copy Location/Pages: E-Copy Location/File Name: P 3.5 Process Validation and/or Evaluation Hard Copy Location/Pages: E-Copy Location/File Name: Please check appropriate boxes. Development Pharmaceutics Report Starting page #: Ending page#: Validation Scheme Starting page #: Ending page#: ____ (e.g. 2) Pilot batches were used in the validation study Starting page #: Ending page#: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 13 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Please check appropriate boxes. ____ (e.g. 3) full production batches were used in the validation study Starting page #: Ending page#: Type of Validation Retrospective Prospective Concurrent* Others; please specify: * Prior consultation with HSA is required. Manufacturing site at which the validation is carried out: Product formula of validation batches: Batch Number Date of Production Same as section P.3.2 Yes No, please provide justification Batch Size Batch Type (production/pilot/experimental) Post-Approval Commitment (1) Validation protocol for commitment batches: Protocol Parameter Description Number of batches per strength Batch Size P 4 CONTROL OF EXCIPIENTS P 4.1 Specifications Specifications for non-compendial excipients and for compendial excipients which include supplementary tests not required by the monograph(s) may be found in: Hard Copy Location/Pages: E-Copy Location/File Name: P 4.2 Analytical Procedures HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 14 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Hard Copy Location/Pages: E-Copy Location/File Name: P 4.3 Validation of Analytical Procedures Hard Copy Location/Pages: E-Copy Location/File Name: P 4.4 Justification of Specifications Justification of the specifications (e.g., evolution of tests, analytical procedures, and acceptance criteria, exclusion of certain tests, differences from compendial standard, etc.): Hard Copy Location/Pages: E-Copy Location/File Name: P 4.5 Excipients of Human or Animal Origin Hard Copy Location/Pages: E-Copy Location/File Name: P 4.6 Novel Excipients Hard Copy Location/Pages: E-Copy Location/File Name: P 5 CONTROL OF DRUG PRODUCT P 5.1 Specification(s) Standard Claimed for the Drug Product (e.g., USP, Ph.Eur, BP, JP etc.): Test Method (e.g., Source (e.g., HPLC) USP, In-house) HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Release Specification Shelf Life Specification Appendix 8 - Page 15 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Standard Claimed for the Drug Product (e.g., USP, Ph.Eur, BP, JP etc.): Test Method (e.g., Source (e.g., HPLC) USP, In-house) Release Specification Shelf Life Specification P 5.2 Analytical Procedures P 5.3 Validation of Analytical Procedures For each test, please indicate “yes” or “no” as appropriate System Suitability Or Others (Please specify) Robustness Limit of Quantitation Limit of Detection Precision - Repeatability - Intermediate Precision - Reproducibility Accuracy Range Linearity Selectivity Method Description Test Name (as per P5.1) P 5.4 Batch Analyses Batch Number Batch Size Batch Type* Date of Production Site of Production Site of Batch Release * describe purpose of batch – e.g. developmental, pilot, production, clinical, validation, commercial P 5.5 Characterisation of Impurities HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 16 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Information on the characterization of impurities, not previously provided in S 3.2 (e.g., summary of actual and potential degradation products, basis for setting the acceptance criteria, etc): Chemical Name/Laboratory Code Origin/Type of Impurity P 5.6 Justification of Specification(s) Hard Copy Location/Pages: E-Copy Location/File Name: Test Justification of Specifications P 6 REFERENCE STANDARDS OR MATERIALS If the reference standard is a secondary standard (in house /working standard), evidence that the secondary standard has been standardised against an official standard should be provided. Data of studies performed on working standard against primary standard should be included, together with appropriate Certificate of Analysis. Hard Copy Location/Pages: E-Copy Location/File Name: Drug Substance Batch Number Source (e.g., USP, in-house) Batch Number Source (e.g., USP, in-house) Primary Reference Standard Working Standard Impurities Primary Reference Standard Working Standard P 7 CONTAINER CLOSURE SYSTEM Description of the container closure systems: Description of Container Closure Quantity Per Container HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Pack Size Appendix 8 - Page 17 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Description of the container closure systems: Description of Container Closure Quantity Per Container Pack Size P 8 STABILITY P 8.1 Stability Summary and Conclusions Hard Copy Location/Pages: E-Copy Location/File Name: Proposed Commercial Batch Size (kg): Same as section P.3.2 Yes No, please provide justification Product formula of Stability Batches Batch Number Batch Size Storage Conditions (°C, % RH, light) Date of Manufacture Site of Manufacture Batch Number Source of Active Ingredient and Batch Number Container Closure System Completed Test Intervals E.g. 0, 3, 6, 9, 12, 18, 24, 36 months In-use stability testing (where applicable): In-use Storage Conditions (°C, % RH, light) Length of Storage prior to Start of In-use Stability Testing HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Completed In-use Test Intervals (e.g. minutes/ hours/ days) Appendix 8 - Page 18 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Proposed storage conditions and shelf life: Container Closure System Storage Conditions (with In-use Shelf Life (with In-use Period, Storage Conditions, if applicable) if applicable) P 8.2 Post-Approval Stability Protocol and Stability Commitment Hard Copy Location/Pages: E-Copy Location/File Name: (1) Stability protocol for commitment batches: Protocol Parameter Description Number of batches per strength and batch sizes Tests and acceptance criteria Container closure system(s) Testing frequency Storage conditions (and tolerances) of samples Other (2) Stability protocol for continuing (i.e., ongoing) batches: Protocol Parameter Description Number of batches per strength per year and batch sizes Tests and acceptance criteria Container closure system(s) Testing frequency Storage conditions (and tolerances) of samples Other P 8.3 Stability Data HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 19 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 Hard Copy Location/Pages: E-Copy Location/File Name: P 9 PRODUCT INTERCHANGEABILITY P 9.1 Bioavailability / Bioequivalence Study Generic Product Submitted to HSA for Registration Current Registered Singapore Reference Product Product Name Strength of Dosage Form Site of Manufacture Site of Batch Release N/A Details of BA/BE Study: Study Report Number BA/BE Study Site (Name & Address) Date of Inspection of Study Name of Inspecting Agency/Authority Availability of Inspection Report (Yes/No) Generic Product Used in BA/BE Study Reference Product Used in BA/BE Study Product Name Strength of Dosage Form Site of Manufacture Site of Batch Release N/A Country where the supply is sourced for this study: Batch No. Batch size Product formula N/A Same as section P.3.2 Yes No, please provide justification N/A P 9.2 Comparative Dissolution Profile Hard Copy Location/Pages: E-Copy Location/File Name: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 20 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 State the Product Name & strength, batch number and either BE/SIN reference product or generic product. Product 1: = Product 2: = Study Report Number: Profile of Product 1 Profile of Product 2 Product Name Strength of Dosage Form Site of Manufacture Site of Batch Release Country where the supply is sourced for this study: Description of Dissolution Method Used Dissolution Test Results e.g. USP paddle 1, 900mL, 50rpm Profile of Product 1 *0min 15min 30min 45min Profile of Product 2 60min 0min 15min 30min 45min 60min Medium 1 Range Mean of 12 tablets RSD F2 Calculation Medium 2 Range Mean of 12 tablets RSD F2 Calculation Medium 3 Range Mean of 12 tablets RSD F2 Calculation Location of Dissolution Graphs *Please revise the table accordingly to suit the number of testing time intervals used. HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Appendix 8 - Page 21 of 22 GUIDANCE ON MEDICINAL PRODUCT REGISTRATION IN SINGAPORE – SINGAPORE QUALITY OVERALL SUMMARY FOR CHEMICAL DRUGS APRIL 2011 A APPENDICES A 1 FACILITIES AND EQUIPMENT (NAME, MANUFACTURER) Hard Copy Location/Pages: E-Copy Location/File Name: A 2 ADVENTITIOUS AGENTS SAFETY EVALUATION (NAME, DOSAGE FORM, MANUFACTURER) Hard Copy Location/Pages: E-Copy Location/File Name: A 3 NOVEL EXCIPIENTS Hard Copy Location/Pages: E-Copy Location/File Name: Applicant’s Name: HEALTH SCIENCES AUTHORITY – HEALTH PRODUCTS REGULATION GROUP Date: Appendix 8 - Page 22 of 22









