Technique #1 – Use of a Burette
advertisement

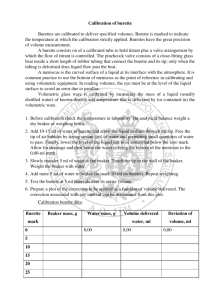
CHEM 2204 – Chemical Laboratory Techniques Practical Lab Techniques Datasheets Technique #1 – Use of a Burette Technique #2 – Use of a Pipette Technique #3 – Use of a Volumetric Flask Technique #4 – Weighing Technique #5 – Use of a Bottle-Top Dispenser Technique #6 - Gravity Filtration, Vacuum Filtration Technique #7 – Preparation of a Standard Solution Technique #8 – Titration Instructions to students: Students should refer to the “Demonstrations of the Eight Practical Lab Techniques” document when attempting to complete all the tasks outlined for each technique. By the end of the lab, it is expected that you will be able to carry out all the tasks using the proper chemical laboratory techniques. Study the photos and diagrams carefully. Pay close attention to the assembly of the apparatus and how each piece of glassware is positioned relative to each other. If you are uncertain about a technique, consult your instructor. When recording data on the datasheet, record the data directly in pen, and record the data using the proper number of significant figures. Jan 04, 2011 Page 1 Technique #1 – Use of a Burette Task: 1. Prepare and fill the burette with the solution provided. 2. Record the initial burette reading. 3. Place an Erlenmeyer flask under the burette to receive the solution from the burette. 4. Using the timer provided, open the stopcock for approximately 15 seconds. 5. Record the final burette reading. 6. Determine the volume of the solution delivered into the Erlenmeyer flask. Datasheet: 1. Final Burette Reading ______________________ 2. Initial Burette Reading ______________________ 3. Volume of solution delivered ______________________ Jan 04, 2011 Page 2 Technique #2 – Use of a Pipette Task: 1. Choose a volumetric pipette from the selection of volumetric pipettes available. 2. Determine the volume that the volumetric pipette can hold. 3. Using the pipette chosen, transfer the volume of a solution provided into an Erlenmeyer flask. 4. Enter the volume of the solution that has been transferred into the beaker in the datasheet. Datasheet: Volume of the solution in the beaker Trial 1: ______________________ Trial 2: ______________________ Trial 3: ______________________ Jan 04, 2011 Page 3 Technique #3 – Use of a Volumetric Flask Solutions are often prepared by dilutions of a stock solution. The concentration of the stock solution, Ci, is given on the reagent bottle. The dilution equation is CiVi = CfVf Equation (1) where Ci = concentration of the stock solution Vi = volume of the stock solution Cf = final concentration of the dilute solution Vf = final volume of the dilute solution The dilution factor, D is D = Ci / C f . Equation (2) Example: Prepare a 100.0 mL of a solution by diluting the stock solution provided by a dilution factor of 30. The concentration of the stock solution is 2.010 M. To calculate the final concentration of the dilute solution, let Ci=2.010 M and let D= 30. Substitute Ci and D into equation (2) and solve for Cf, the concentration of the dilute solution. 30 = 2.010 M / Cf Cf = 2.010 M / 30 = 0.06700 M The concentration of the dilute solution is 0.06700 M. Use Equation (1) to determine the volume of the stock solution, Vi, needed. (2.010 M) Vi = (0.06700 M) (100.0 mL) Vi = 3.333 mL Planned action: To prepare a 0.4020 M solution, withdraw 3.333 mL from the 2.010 M stock solution and transfer this quantity into a 100 mL volumetric flask. Make up to the mark with distilled water and mix thoroughly. Jan 04, 2011 Page 4 Task: 1. You are asked to dilute the stock solution, Solution A, by the dilution factors given in the table below. Each diluted solution has a final volume of 100.0 mL. The Stock solution, Solution A, is 2.010 M. Using Equations (1) and (2), calculate Cf and Vi. Fill out the table below. Dilution Factor Ci (M) Cf (M) Vi (mL) Vf (mL) 2 2.010 _______ _______ 100.0 5 2.010 _______ _______ 100.0 10 2.010 _______ _______ 100.0 2. Reach your hand into the box and draw a dilution factor. 3. Using the calculated values, prepare 100.0 mL of the dilute stock solution. Your Planned action: | To prepare a _____________ M (Cf) solution, withdraw ___________ mL (Vi) from the 2.010 M stock solution and transfer this quantity into a 100 mL volumetric flask. Make up to the mark with distilled water and mix thoroughly. Jan 04, 2011 Page 5 Technique #4 – Weighing Task: Weigh a sample of sand 1. Weigh by taring a sample of sand that is approximately 0.5 g. Transfer the sand sample into a beaker. 2. Enter your mass measurements into the datasheet. 3. When you are finished, return the sand to the bottle provided and wipe the beaker free of sand using a kimwipe. 4. Weigh by difference a sample of sand that is approximately 0.5 g. (Note: In this step, recall what 0.5 g of sand look like from Step 1. Use a spatula and transfer approximately 0.5 g of sand into the weigh boat by estimating the amount visually. You will not be using a top-loading balance.) Transfer the sand sample into a beaker. 5. Enter your mass measurements into the datasheet. 6. When you are finished, return the sand to the bottle provided and wipe the beaker free of sand using a kimwipe. Datasheet: Weighing by taring 1. Mass of weigh boat and sample 2. Mass of empty weigh boat 3. Mass of sample ______________________ 0.0000 g ______________________ Weighing by difference 1. Mass of weigh boat and sample ______________________ 2. Mass of emptied weigh boat ______________________ 3. Mass of sample transferred ______________________ Jan 04, 2011 Page 6 Technique #5 – Use of a Bottle-Top Dispenser Task: 1. Reach your hand into the box and draw a volume. Preset the bottle-top dispenser to this volume. 2. Use a graduated cylinder and measure the volume dispensed by the bottle-top dispenser. The volume collected should be within 2% of the preset volume. 3. If the volume being dispensed is outside the preset volume, adjust the dispenser and repeat step 2. 4. Enter the preset volume and the volume dispensed in the datasheet. Datasheet 1. Preset dispenser volume _________________________ 2. Volume dispensed by the bottle-top dispenser Trial 1: __________________ Trial 2: __________________ Trial 3: __________________ Jan 04, 2011 Page 7 Technique #6 – Gravity Filtration Task: 1. Set up the gravity filtration apparatus (Refer to Figure 22). 2. Prepare the filter paper (Refer to Figure 21). 3. Obtain a sample of sand/water mixture. The mixture contains soluble contaminants. 4. Wash and filter the sand using decanting, and washing techniques. 5. Finally, transfer the washed sand to the funnel. Space to make notes: Jan 04, 2011 Page 8 Technique #6 – Vacuum Filtration Task: 1. Set up the gravity filtration apparatus (Refer to Figure 22a). 2. Prepare the filter paper. 3. Obtain a sample of sand/water mixture. The mixture contains soluble contaminants. 4. Wash and filter the sand using decanting, and washing techniques. 5. Finally, transfer the washed sand to the funnel. Space to make notes: Jan 04, 2011 Page 9 Technique #7 – Preparation of a Standard Solution Task: You will be asked to prepare a 50.00 mL standard solution of copper (II) sulfate pentahydrate, CuSO4•5H2O. 1. Reach your hand into the box and draw a concentration. This is the concentration of the CuSO4•5H2O standard solution you are preparing. 2. Calculate the mass of CuSO4•5H2O needed using this equation. 3. a. Concentration of CuSO4•5H2O standard solution b. Molar mass of CuSO4•5H2O c. Total volume of solution d. Mass of CuSO4•5H2O to be weighed __________________ __________________ 50.00 mL ________________ 4. Weigh the CuSO4•5H2O sample. Weighing by taring 1. Mass of weigh boat and CuSO4•5H2O 2. Mass of empty weigh boat 3. Mass of CuSO4•5H2O Jan 04, 2011 _______________________ 0.0000 g ______________________ Page 10 Technique #8 – Titration Task: Carry out an acid-base titration 1. The burette is already acclimatized with the base. Use a clean and dry beaker to obtain a small quantity of acid. 2. Pipette 10.00 mL of the acid into an Erlenmeyer flask. 3. Add a few drops of phenolpthalein. 4. Perform an acid-base titration. 5. Repeat the titration until you are satisfied with your technique. Your titration volume should be within 0.06 mL or less. Datasheet for a Titration Trial 1: 1. Final burette reading ______________________ 2. Intitial burette reading ______________________ 3. Volume of titrant used in titration ______________________ Trial 2: 1. Final burette reading ______________________ 2. Intitial burette reading ______________________ 3. Volume of titrant used in titration ______________________ Trial 3: 1. Final burette reading ______________________ 2. Intitial burette reading ______________________ 3. Volume of titrant used in titration ______________________ Jan 04, 2011 Page 11