DEHYDRATION OF 2-METHYLCYCLOHEXANOL
advertisement

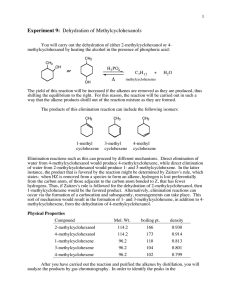
Chem 323 B. Terem DEHYDRATION OF 2-METHYLCYCLOHEXANOL CH3 CH3 OH bp. 165-168oC H3PO4 CH3 + + bp. 110oC CH2 bp. 104oC bp. 102oC trace Alkenes can be easily prepared from alcohols by acid-catalyzed dehydration (i.e. elimination of water, H+ and OH-, to form a double bond).1 When the alcohol is unsymmetrically substituted, as in the present case, a mixture of isomeric alkenes usually results, in which the most stable one usually predominates2. For this reason other (generally more difficult and expensive) synthetic methods are used, whenever a single, pure isomer is desired. Almost any strong Bronsted acid will catalyze elimination of water from secondary and tertiary alcohols, as will many Lewis acids, such as BF3 and I2. Sulfuric acid is too strong of an acid and will likely isomerizes the alkene products (via re-protonation of the C=C bond); for this reason a weaker proton donor such as phosphoric acid is used. In the present experiment 2-methylcyclohexanol is dehydrated to a mixture of alkenes. After the removal of co-distilled water and traces of acid, the overall yield is calculated. It is possible to determine the ratios of the isomeric products through further analysis, such as gas chromatography. Experimental Procedure: To a 50 or 100 mL round bottomed flask add 8.0 mL of 2-methylcyclohexanol, 3 mL of phosphoric acid, and a few boiling chips. Clamp the flask in a heating mantle and assemble the appropriate glassware for fractional distillation (see PLK Fig. 7.2, p 567). Don’t forget to lubricate the joints. Use “Keck” clips between the condenser and the distillation head, as well as between the condenser and the bent adaptor. Heat the flask slowly and evenly in the heating mantle until the product distills out. The boiling point of the distillate should be kept below 96oC by regulating the rate of heating3. The efficiency of fractional distillation cannot be maintained if the reaction flask is heated intermittently or unevenly. Continue distilling until 6-8 mL of liquid have been collected, but stop before the reaction vessel is dry. Check the collecting flask to see if you have a two-phase distillate. (Alkenes have lower densities than water; therefore, the lower layer is water). If the amount of distillate is very small, remove the water with a Pasteur pipette. Transfer the (two-phase) distillate to a small (125 mL) separatory funnel and remove the water layer (if you have not already done so). Wash the upper organic layer with about 3 mL of 10% sodium carbonate. Drain the aqueous sodium carbonate (lower layer). Wash the remaining organic layer with 5 mL of saturated brine (NaCl solution). Drain the brine (lower layer). Transfer the organic liquid in the separatory funnel into a preweighed vial Chem 323 B. Terem (make sure that you record the actual weight of the vial) using a Pasteur pipette (Transferring a liquid from a separatory funnel using a pipette is somewhat unusual; however, it works better in this case with the small quantities being handled). Determine the weight of the product in the vial. Add the appropriate drying agent into the vial. Label your vial and keep it in your locker. It is best to place the vial in a small beaker. Notes: 1 See Smith, Sect 9.8; 2See Smith, Sect.8.5 p. 291-3 If the head temperature exceeds 96oC, the distillate may contain measurable amounts of 2-methylcyclohexanol, which will invalidate the yield calculations. 3 Questions: If possible, answer these questions before leaving the lab (you might start thinking about them while the experiment is going); discuss your answers with the instructor. 1. 2. 3. The 2-methylcyclohexanol used in this experiment is actually a mixture of two stereoisomers. Draw their structures (in a way they can be distinguished from each other) and name them. Write a step-wise mechanism for the phosphoric acid catalyzed dehydration of 2-methylcyclohexanol, showing how both major and minor products can be formed. Show how a tertiary carbocation can be formed by the reprotonation of the major reaction product, and show how this can lead to methylenecyclohexane. How might this acid-catalyzed isomerization be minimized experimentally (already mentioned in the hand-out)