Quiz 2 Review Sheet
advertisement

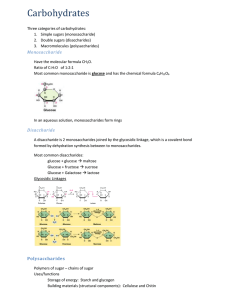
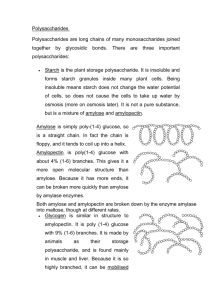
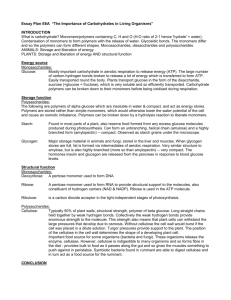
Exam 2 Review Sheet Honors Biology USE THIS SHEET AFTER YOU HAVE STUDIED AS A METHOD OF EVALUATING WHAT YOU STILL NEED TO WORK ON. Exam 2 will cover: Chapter 2 – 2.15 to the end Chapter 3 – Intro through the end of proteins (STOP HERE…have you studied yet? If yes, continue… If no, GO STUDY FIRST! DO NOT USE THIS TO STUDY!) A. Quiz 5 review sheet. B. Download and print out the macromolecule chart on the website. Use this chart as a study tool. 1. Read section 3.19. At least one question will come from this section. 2. Explain on the molecular level what happens to a crystal of glucose when you throw it in water on the molecule level (don’t just say that it dissolves). 2. Compare and contrast organic compounds with hydrocarbons. Why are all hydrocarbons organic, but not all organic molecules are hydrocarbons? 3. Be able to identify/circle the carbon skeleton of a hydrocarbon. 4. Describe what structural isomers are and be able to draw an example. 5. Know the table on page 35 in addition to the sulfhydryl and phosphotidyl groups. 6. Identify the functional groups in the molecule below (there are three): 7. Compare and contrast aldehydes to ketones. Why are these NOT considered functional groups, but rather a type of compound? What is the functional group found in each of these? Which class of macromolecule contains both types of compounds? 8. Which types of compounds usually end in –ol? What functional group is involved? 9. Describe in detail why carboxylic acids are considered acids (You will need to discuss electronegativity differences between atoms). What class of macromolecules contain this functional group? 10. Compare and contrast the carbonyl to the carboxyl group. Which two functional groups add to be a carboxyl? Reminder: if the group is a ccarboxy, it is not also a carbonyl and a hydroxyl (You cannot circle the C=O in question 6 and say it’s a carbonyl. Why?) 11. Know the table on page 35 in addition to the sulfhydryl and phosphotidyl groups. 12. Compare hydrophobic and hydrophilic. What makes something hydrophobic or hydrophilic? Describe how this works on the MOLECULAR LEVEL. Don’t just say nonpolar or polar… Speak in terms of electronegativity and the electromagnetic force. 13. What is meant by a macromolecule? List each and its general function in organisms. 14. Compare monomers to polymers. 15. Explain the reactions in detail used to build and break the macromolecules (Know Figures 3.3A and B). – you should be able to draw these using spheres. 16. Compare and contrast monosaccharides, disaccharides and polysaccharides. In addition, be able to give examples of each. All together these are called what? 17. Be able to draw a chart indicating the various combinations of monosaccharides and the respective disaccharide that forms (maltose, sucrose, lactose). 18. Can you draw the RING structure of glucose yet… (Figure 3.4C) 19. Describe how two glucose molecules join together and the type of linkage that is formed (Figure 3.5). You should be able to draw this chemical reaction. 20. What does the word “carbohydrate” mean, literally and why do we use this word to describe the kinds of molecules that we do? 21. Discuss what is meant by the empirical formula [CH2O]n 22. Look at the structure of ANY monosaccharide. What functional group is attached to every carbon except one? What functional group is that other carbon a part of? 23. If I told you to draw C6H12O6, what would your response be to me… 24. Explain how artificial sweeteners can actually be sweeter than glucose (section 3.6). 25. Compare and contrast the three polysaccharides discussed in class (Figure 3.7). Include their structures, why they have the same or different structures, where they are found in the world, their functions in organisms, how structure relates to function in this case, etc… 26. Cellulose is considered “fiber”. Like when the commercials on TV say that Cheerios is high in “fiber”. What is fiber? Why is cellulose called or at least part of what we call “fiber”? (Yes, you need to look this up) 27. Why are most animals not able to hydrolyze cellulose, but can hydrolyze glycogen and starch when all three are just polymers of glucose? How are some animals able to hydrolyze cellulose? 28. Explain what happens to plant starch when you eat it (like if you eat a potatoe). 29. What is the monomer of the protein, how many are there, what makes each of these different, how are they similar and how are they grouped (the three groups)? Can you draw two of them yet and connect them via dehydration synthesis (condensation reaction)?...you need to be able to 30. Where does the “amino acid” get its name from? 31. Why do I feel that proteins are the most important class of macromolecules (they are all important of course)? 32. Compare an amino acid, dipeptide and polypeptide. 33. Describe how a dipeptide is formed (Figure 3.13). What do we call the bond that links them? How is this analogous to what happens with carbohydrates? 34. What determines the structure of a particular protein? Explain why. Of course, the structure then determines the… 35. Explain why a change in the DNA might lead to problems. 36. Describe in general terms what happens to a polypeptide (or any polymer for that matter) when you eat it. What happens to it, what do you do with it, etc… 37. Describe the four levels of protein structure (Figure 3.15-3.18). I could ask another 15 questions about each level… Know all the details we went over in class like when the side chains come into play or when only the backbone is involved, hydrophobic vs hydrophilic side chains, etc… 38. Compare and contrast how primary, secondary, tertiary and quaternary structure are held together (stabilized). 39. Be able to identify the various secondary structure elements in a protein and circle them if presented with a ribbon diagram. 40. Explain how a ribbon diagram is generated. What are you looking at when you view a ribbon diagram of a protein? 41. Compare and contrast a polypeptide to a protein. When are they the same and when would they be different? 42. What is meant by a subunit in a protein? 43. Explain what is meant by denature and renature. 44. Know the functions of proteins. Use the PowerPoint table (because I do not see them specifically in the book). Know SOME of the examples like hemoglobin, myosin, insulin, insulin receptor, ATP synthase, photosystem II, antibodies, HIV protease, etc… and under what category they would fall. You can google any of these proteins (especially XDH!) and learn the job that it does, which would allow you to place it in one of these groups. 45. How small is a protein relative to the size of a cell? (use the analogy) How many hemoglobin proteins are packed into a single red blood cell? How many red blood cells do you have on average? How many oxygens can your blood carry if it were completely saturated with oxygen? 46. Why are some people considered to be lactose intolerant? 47. Are glycogen, starch and cellulose the only three polysaccharides found in organisms? 48. What is the fate of glucose? Basically, what do organisms use glucose monomers for? Why do we store them? Where do humans store them? 49. If there is too much glucose in your blood, how does your body handle this? Where does it end up? Where might this surge in glucose have come from? 50. For every polymer we eat (disaccharides, polysaccharides, polypeptides, etc…), we need to have a ___________________ somewhere in our digestive tract to hydrolyze it. This may seem obvious, but we eat cells (unless you are eating processed food, which case the cells have already been destroyed and broken apart) of other organisms. There we eat macromolecule or at least polymers for the most part. We need to hydrolyze them. Why? Why not just keep them together? 51. Know who Linus Pauling was and what he did…(section 3.19)