Molality Another unit of concentration that can be used for solutions
advertisement
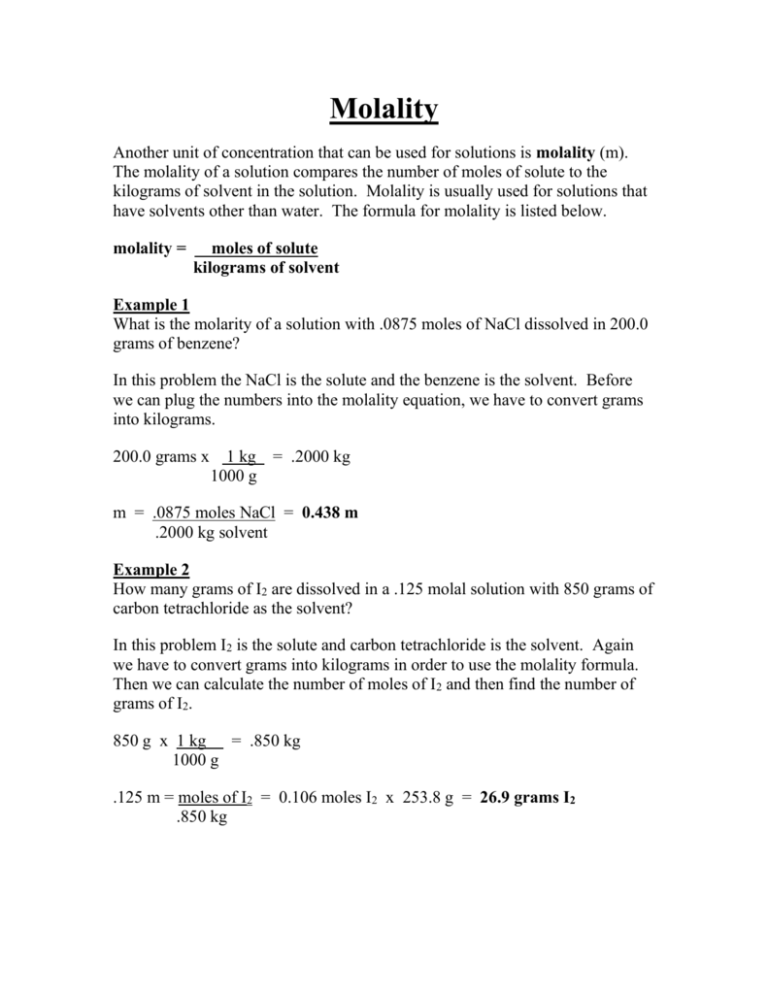
Molality Another unit of concentration that can be used for solutions is molality (m). The molality of a solution compares the number of moles of solute to the kilograms of solvent in the solution. Molality is usually used for solutions that have solvents other than water. The formula for molality is listed below. molality = moles of solute kilograms of solvent Example 1 What is the molarity of a solution with .0875 moles of NaCl dissolved in 200.0 grams of benzene? In this problem the NaCl is the solute and the benzene is the solvent. Before we can plug the numbers into the molality equation, we have to convert grams into kilograms. 200.0 grams x 1 kg = .2000 kg 1000 g m = .0875 moles NaCl = 0.438 m .2000 kg solvent Example 2 How many grams of I2 are dissolved in a .125 molal solution with 850 grams of carbon tetrachloride as the solvent? In this problem I2 is the solute and carbon tetrachloride is the solvent. Again we have to convert grams into kilograms in order to use the molality formula. Then we can calculate the number of moles of I2 and then find the number of grams of I2. 850 g x 1 kg = .850 kg 1000 g .125 m = moles of I2 = 0.106 moles I2 x 253.8 g = 26.9 grams I2 .850 kg








