Phosphorylase Kinase
advertisement

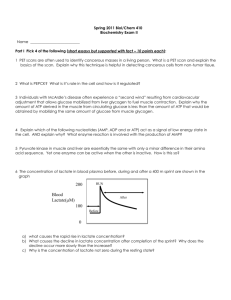
Phosphorylase Kinase Converts phosphorylase b phosphorylase a High abundance protein (0.5% in muscle) Native MWt 1.3 x 106 Da 4 different subunits: probably 444 4 -subunit (45 kDa) has the full protein kinase activity subunits (140 and 120 kDa, respectively) are inhibitory subunits unless phosphorylated by Protein Kinase A -subunit is the catalytic subunit which catalyses the phosphorylation of phosphorylase b at Ser14 -subunit is calmodulin which confers Ca2+ sensitivity to the phosphorylase kinase – the enzyme becomes activated when [Ca2+]cyt increases e.g. in muscle contraction Calmodulin monitors the Ca2+ levels in the cytoplasm can exist as a free form or as a subunit of a heteromeric protein is a highly conserved protein which acts as a Ca2+ activated switch in many organisms regulating many different cell functions it has 4 Ca2+ binding sites per mol with a Kd (dissociation constant) of ~ 1 µM – consistent with the levels of Ca2+ in contracting muscle participates in numerous regulatory processes, including muscle contraction and phosphorylase kinase activation the binding of Ca2+ induces a conformational change in calmodulin, exposing a Methionine-rich, hydrophobic domain which in turn, can then bind to and alter the catalytic function of the -subunit and other regulatory proteins. Physiological significance in muscle muscle contraction is stimulated by a transient increase in [Ca2+]cyt from 0.1 µM to 1 – 10 µM the rate of glycogen breakdown (and thus the provision of ATP by anaerobic glycolysis) is linked to muscle contraction for phosphorylase kinase to be fully activated the subunits must also be phosphorylated by PKA, which, in turn, is activated by adrenaline via cAMP in muscle D Davies BS2510 2003 Protein Phosphatase 1 (PPase 1) reverses the effect of protein kinases on glycogen metabolism the steady-state activity of many regulatory enzymes depend on the fraction of the enzyme in the phosphorylated and dephosphorylated states this is in turn dependent on the relative activities of protein kinases and protein phosphatase about 1/3 of all mammalian proteins have covalently-bound phosphates which may impact on some aspect of their regulation there are about 1,000 different protein kinases encoded in the human genome and about 500 different protein phosphatases Protein Phosphatase nomenclature is rather arbitrary, based mainly on the order in which the have been discovered: the most important form as far as glycogen metabolism is concerned is PPase 1, with a lesser role played by PPase 2A These enzymes hydrolyse specific phosphate residues covalently bound to Serine or Threonine, thus reversing the effects of the protein kinases PPase 1 brings about the dephosphorylation and inactivation of Phosphorylase a and of phosphorylase kinase (subunits) and the dephosphorylation and activation of Glycogen Synthase. Regulation of PPase 1 In turn the activity of PPase 1 is regulated by two different proteins PPase Inhibitor 1 (Inhibitor 1) and G-subunit Inhibitor 1 Inhibitor 1 (found in both liver and muscle) is yet another example of a protein regulated by PKA and PPase 1 although in this case the protein is phosphorylated on a Threonine residue The protein is only functional (i.e. inhibitory) when it is phosphorylated. It inhibits PPase 1 The concentration of cAMP in the cell controls the fractions of Phosphorylase and Glycogen Synthase which are in the active form by activating PKA and by inhibiting PPase 1. The two effects reinforce one another. G-subunit G-subunit (Glycogen-targeting Subunit) occurs in different forms in muscle (160 kDa) and liver (33 kDa) ( nothing to do with heterotrimeric, plasma membrane associated G-proteins involved in signalling!) G-subunits target PPase 1 to glycogen and therefore to the enzymes of glycogen metabolism One form of PPase 1G is far more active than other forms, this consist of the catalytic subunit and the G-subunit Protein kinase A can also phosphorylate G-subunit leading to the release of PPase 1 into the cytosol and the inactivation of the enzyme is completed by Inhibitor 1. The reason for this mechanism is probably to prevent the uncontrolled dephosphorylation of cytosolic proteins by PPase 1 – the enzyme is only active when associated with the Glycogen The dissociation of the G-subunit/PPase 1 complex is dependent on the phosphorylation of G-subunit at site 1 and this is reversed by PPase 2A. This occurs quickly once the adrenalin has exerted its desired effect, G-subunit can recombine with the catalytic subunit, PPase 1 is activated and GS is activated, phosphorylase inactivated and glycogen synthesis can recommence In times of plenty, insulin stimulates Glycogen Synthase via the activation of an insulin-stimulated protein kinase which leads to G-subunit being phosphorylated at site 2, which results in the activation of PPase 1 and Glycogen synthesis occurs. Regulation of Glycogen Metabolism in the liver There are many points of similarity with muscle where these have been studied and some differences reflecting the different function of glycogen metabolism in the two tissues(See below) G-subunit is much smaller (33kDa, 284 aa), binds PPase 1 at a sequence RVSF near the N-terminal, binds glycogen at residues 144-231, and phosphorylase a near the C-terminal. But there is no association with the ER and G-subunit is not regulated by phosphorylation/dephosphorylation in liver. PP1 Glycogen 56 –64 144-231 Phosphorylase a 269-284 Glucagon mediates increased cAMP in liver, adrenalin and noradrenalin work via changes in IP3 and Ca2+. Liver glycogen is used to buffer blood glucose. The liver has a specific Glucose-6-phosphatase and Glucokinase to regulate uptake and export of the sugar. When glucose is infused into the liver, glycogen synthesis only starts after glycogen degradation has stopped. Similarly when cAMP is infused Glycogen Synthase is only switched off when phosphorylase has been fully activated. In the liver PPase 1 is strongly bound to phosphorylase a. When glucose level rises, glucose binds to phosphorylase a ,this changes its conformation making it a better substrate for PPase 1. This results in the inactivation of phosphorylase and the release of PPase 1 which can then dephosphorylate and activate glycogen synthase