Lewis Structures: Organic Chemistry Lecture Notes
advertisement
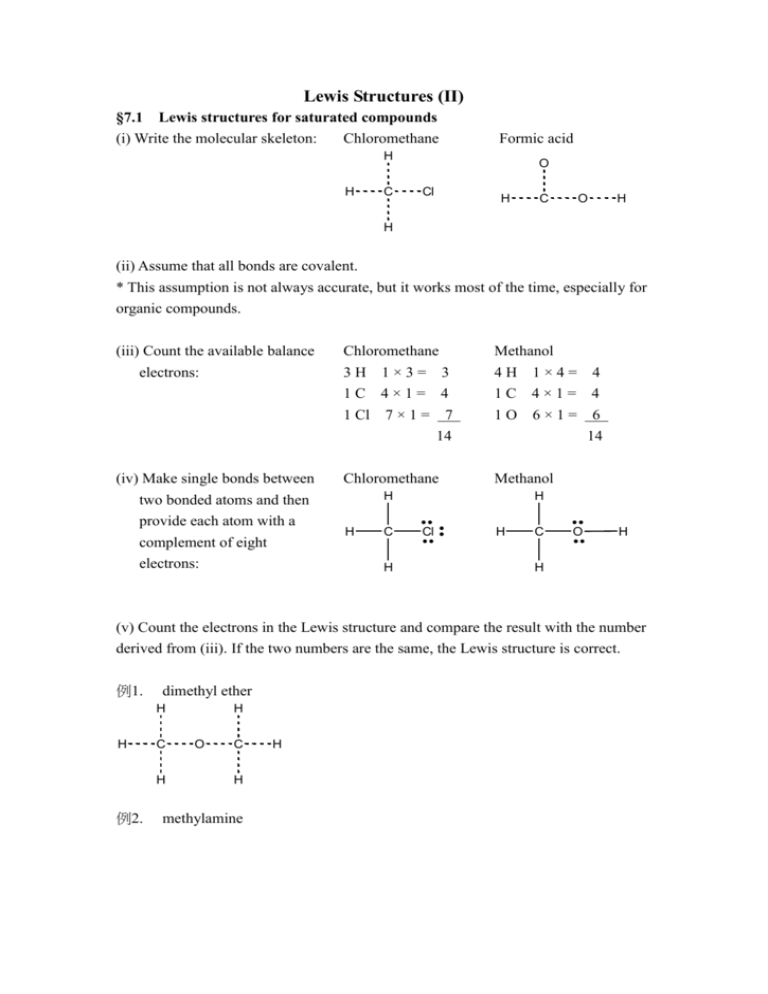
Lewis Structures (II) §7.1 Lewis structures for saturated compounds (i) Write the molecular skeleton: Chloromethane Formic acid H H C O Cl H C O H H (ii) Assume that all bonds are covalent. * This assumption is not always accurate, but it works most of the time, especially for organic compounds. (iii) Count the available balance electrons: (iv) Make single bonds between two bonded atoms and then provide each atom with a complement of eight electrons: Chloromethane 3H 1×3= 3 Methanol 4H 1×4= 4 1C 4×1= 4 1 Cl 7 × 1 = 7 14 1C 4×1= 4 1O 6×1= 6 14 Chloromethane Methanol H H C H H Cl H C O H H (v) Count the electrons in the Lewis structure and compare the result with the number derived from (iii). If the two numbers are the same, the Lewis structure is correct. 例1. dimethyl ether H H C H 例2. H O C H methylamine H H H C H N H H 例3. methanethiol H H C S H H 例4. methylal (or dimethoxymethane) H H C H H O C H H O C H H §7.2 Unsaturated groups When the compound in question is unsaturated, step(v) shows that the number of electrons in the trial structure is larger than the number of available valence electrons. For example, ethylene H H H C C H C C H H H H In such case the trial structure cannot be correct, and it must be modified by introducing one or more multiple bonds. (vi) Remove an unshared pair from each of the two adjacent atoms and add a second bond between them. (Each such operation reduces the number of electrons in the trial structure by two.) H H H H C C C H H H 例1. H H C C C H H H formaldehyde H C O H 例2. acetonitrile (We have to do the operation in step(vi) twice to make the number of electrons in the trial structure equal to the number of available valence electrons.) H H C C N H 例3. formic acid The double bond could be placed between the carbon atom and either one of the two oxygen atoms. O H O C H O H O or C O H H C O H The second structure is less acceptable since the oxygen atoms have the unfamiliar valences of one and three respectively. 例4. acetyl chloride H H C O C Cl H Exercises ex1. propyne H H C C C H O H C C C H H ex2. acetone H H H H ex3. formamide O H C N H H ex4. urea O H N H C N H H Exceptions to the octet rule It is not always possible for all molecules to arrange the electrons in pairs. This is most obvious for molecules with an odd number of electrons. For example, ClO2 and NO2 : ClO2 NO2 Cl O N O O bent, partial double bond, and an angle of 117.5o. O bent, partial double bond, and an angle of 134.25o. Molecules which contain such unpaired electron are called radicals, usually categorized reactive species. Following the octet rule, the radical center needs to complete its valence shell by the acquisition of one more electron, for chemical stability. Lewis acids are species containing an atom which is short of a pair of electrons to make an octet. Aluminum trichloride Boron trifluoride Cl Cl F F Al B Cl F Although stable under the right conditions, these Lewis acids are vigorously reactive toward compounds that have electron pairs to give. Foe example, BF3 + N(CH3)3 F F CH 3 B N F CH 3 CH 3 Molecules with such lone pairs are called Lewis bases. The reactions in which Lewis acids react to complete their valence shell are used in organic syntheses to yield unstable intermediates such as the cations CH3+ and Cl+ which are not easily obtained by other routes. AlCl3 + Cl-CH3 AlCl4- + CH3+ AlCl3 + Cl-Cl AlCl4- + Cl+ A particularly interesting example of the Lewis acid/base classification is the family of carbenes CX2. These species are Lewis bases since they have a lone pair on carbon, and also Lewis acids since the carbon atom only has six valence electrons. Cl C Cl Cl C Cl Cl 2C CCl 2 §7.3 Large molecules Organic chemistry is largely the chemistry of functional groups. Often in writing reaction mechanisms it is only necessary to write the Lewis structure of the functional groups; for example, CH 3 H3C C O O O H CH 3 C , H N CH 3 , Most organic textbooks do not include the unshared pairs in functional groups; this is really a unfortunate practice because much of the chemistry of a functional group is determined by the presence (or absence) of unshared electron pairs. In order to write Lewis structures of functional groups attached to any alkyl (or aliphatic, R-) or aryl (or aromatic, Ar-) group, one allows each R- or Ar- attached to the functional group to bring one electron into the union. 例1. phenyl methyl ketone (acetophenone) O C 例2. CH 3 benzyldimethylamine CH 3 H2 C N CH 3 例3. benzaldoxime H C N O Exercises H ex1. furan H H C H C C C H O ex2. azobenzene N N ex3. methyl benzimidate H N C O CH 3 ex4. benzophenone phenylhydrazone H C N N ex5. ethyl crotonate H 3C H H C C O C O H2 C CH 3 ex6. DMSO, dimethylsulfoxide, (CH3)2SO §7.4 例1. Formal charge nitrobenzene The nitrogen atom has a formal charge of +1; the oxygen atom that is bonded to N by a single bond has a formal charge of -1. O N O 例2. pyridine N-oxide N 例3. The nitrogen atom has a formal charge of +1; the oxygen atom has a formal charge of -1. O benzenesulfonic acid O S O O H The sulfur atom has a formal charge of +2; each oxygen atom that is bonded to S by a single bond and has three unshared electron pairs has a formal charge of -1. §7.5 Ions A very number of organic reactions involve ions as intermediates. With very few exceptions the ions encountered in organic chemistry will have a total charge of +1 or -1. The way how ions arise in a reaction involves some sort of bond-breaking or bond-making process. Cations 例1. Consider the molecule of chloromethane is broken at the C-Cl bond so that the two electrons constituting the C-Cl bond depart with Cl: H3C CH3+ Cl + Cl- Organic chemists use curved arrows to indicate the movement of electrons, and this process is called “pushing electrons”. Note that the base of the arrow begins at the original location of the bonding pair of electrons, and that the head of the arrow points to the destination of the electrons-the chlorine atom. When the head of the arrow has two barbs, it denotes the movement of a pair of electrons. If a neutral molecule is cleaved, an excess of electrons (negative charge) will result at the head of the arrow, and a deficient of electrons (positive charge) will result at the tail of the arrow. Two ions, one electron-poor and the other electron-rich, result from the heterolytic (unsymmetrical) bond cleavage. 例2. Consider the result when a proton, H+, becomes bonded to methanol by way of one of the unshared electron pair on O: H+ H3C O H H H3C O H This time the curved arrow is used to signify bond making. A pair of unshared electrons on O is pushed toward the region between the oxygen atom and the hydrogen ion; it becomes an O-H covalent bond. The formal charge distribution on the resulting structure is predictable from the arrow: electrons are pushed away from O, leaving it with a positive charge; electrons are pushed toward the hydrogen ion, neutralizing its erstwhile positive charge. Anions 例1. Consider the result if the C-Li bind of methyl lithium were broken so that the two electrons constituting the bond both remain with the carbon: H H3C H Li C + Li+ H Compare this example with those in (1)Cations; all are heterolytic cleavages. In this example the electrons move toward C, generating a carbanion; conversely, in those examples of (1) the electrons move away from C and produce a carbocation. The original polarity of the bond influences the direction of heterolysis: the more electronegative atom takes the electrons. Free radicals Covalent bonds can suffer hemolytic (symmetrical) bond breaking, yielding radicals with a formal charge of zero. 例1. When di-tert-butyl peroxide breaks homolytically, CH 3 H3C C CH 3 CH 3 O O C CH 3 CH 3 CH 3 2 H3C C O CH 3 Note that the head of the arrow has only one barb signifying the movement of a single electron, and two such arrows are required to denote hemolytic bond breaking. Homolytic bond breaking usually requires quite large energies; many free radical reactions are promoted photochemically. Homolytic cleavage reactions important to organic chemistry often occur when the covalent bond in question is weak; oxygen-oxygen (peroxide) bonds are a good example. 例2. Carbon radicals are usually produced indirectly. An exception is the hemolytic cleavage of azobis(isobutyronitrile): CN H3C C CH3 CN N N C CH3 CN CH3 2 H3C C CH3 + N N




