CM3109
advertisement

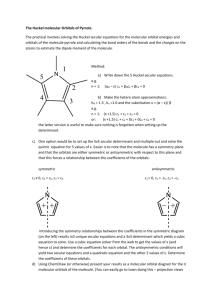
CM3109 Practical Comparison of the calculation of π molecular orbitals by ab-initio (Gaussian 03) computation and by Huckel theory. The purpose of this practical is to demonstrate how to use a full ab-initio (Gaussian 03) computation to calculate the properties of larger molecules, in particular the molecular orbital energies and composition. The test molecules used are planar conjugated hydrocarbons and the results for the π molecular orbitals are compared to those of Huckel theory, thereby estimating the values of the Huckel α and β parameters. Use the Gaussian 03 program to calculate the molecular orbitals of ethene, butadiene and benzene. The input is basically the same as for the earlier calculations (Hartree-Fock, STO-3G basis set, geometry optimized) but this time the output of the molecular orbitals is also requested. This is achieved by including the command POP=Regular instructs the calculation to print out the details of the 5 HOMO and 5 LUMO. The Route Section will read: HF/STO-3G opt pop=regular (The STO-3G is the smallest and least accurate basis set provided by the Gaussian03 suite of programs and is only used here in order to minimize the time taken in the practical.) (The %Section of the input is ignored; the Title is anything you like; the Charge and Multiplicity are 0 1) Larger molecules require the following style of input in the Molecule Specification section: 1st atom 2nd is bonded() to 1st atom at a distance r1 3rd2nd r2, and makes an angle(<) with 1st atom of a2 4th3rd r3, < 2nd a3, and makes a dihedral < with the 1st atom of p3 … (all other atoms have a line similar to the last one) The dihedral angle for planar molecules is 0.0 if atoms 4 and 1 are cis and 180.0 if atoms 4 and 1 are trans. Example of ethene: 1st atom H 2nd bonded() to 1st distance r1 C 1 r1 C 2 r2 1 a2 H 3 r3 2 a3 1 180.0 4th3rd r3 < 2nd a3 trans to 1st H 2 r4 3 a4 4 180.0 5th2nd r4 < 3rd a4 trans to 4th H 3 r5 2 a5 5 0.0 3rd2nd r2 makes angle(<) with 1st of a2 6th2nd r5 < 2nd a5 cis to 5th (blank line) r1 1.1 (This section is the initial values of the distances and angles to be optimized.) r2 1.4 … a1 120.0 a2 120.0 … (blank line) . Input the data, run the calculation, inspect the molecular orbitals and sketch a molecular orbital diagram showing the energies of the molecular orbitals and the sketched composition of the π molecular orbitals. Noting that Huckel Theory gives the energies of the π and π* orbitals as α ± β respectively, estimate values of α and β. Repeat the above for butadiene and benzene. In order to obtain the Huckel Theory orbital energies of benzene (in terms of α and β) and the composition of the orbitals, use the web site: http://www.chem.ucalgary.ca/SHMO/ which is fairly self explanatory. The report should contain the three molecular orbital diagrams with the drawings of the π molecular orbitals and the values of α and β and a orbital compositions from the two computational methods.. A brief discussion on why you think the values of α and β are not exactly the same for the three molecules should be included.