Anaerobic Jar (Brewer's Gas Pak)-
advertisement
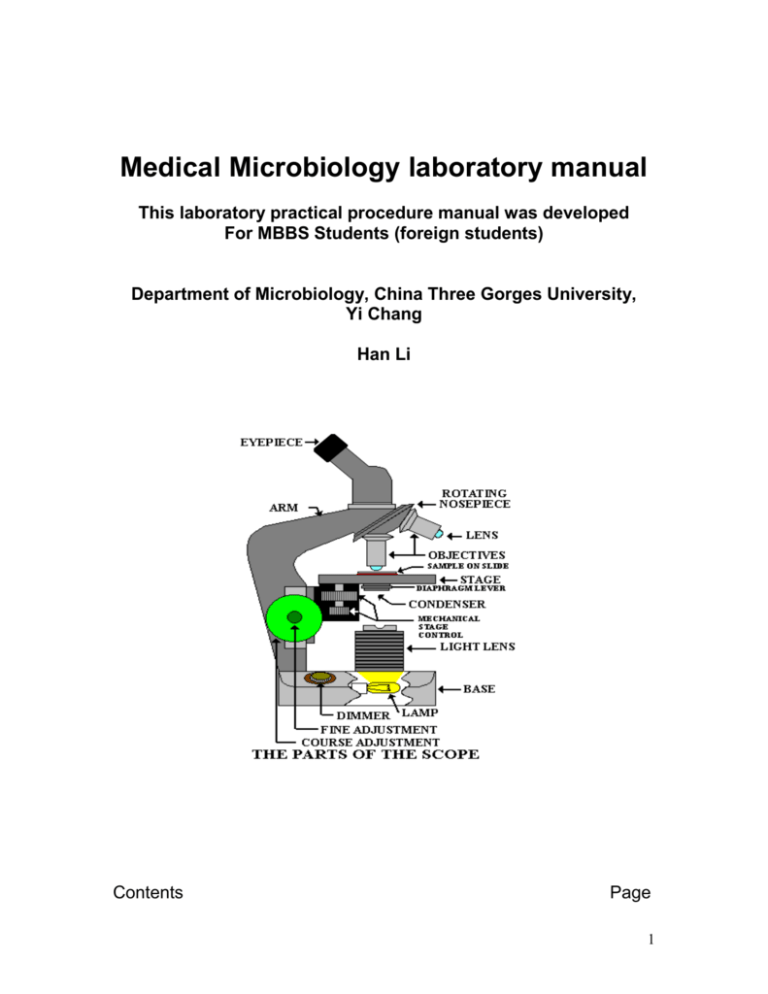
Medical Microbiology laboratory manual This laboratory practical procedure manual was developed For MBBS Students (foreign students) Department of Microbiology, China Three Gorges University, Yi Chang Han Li Contents Page 1 Introduction 4 Generals rules of microbiology laboratory 4 Microscopy Procedure to see the smear 5 Laboratory growth media Types of media 6 Microbiological specimens Specimens rejection criteria Specimen labeling 6 Methods of isolation from clinical specimens Streaking procedure 7 Candle jar technique 9 Method of culture of anaerobes 10 Bacterial colony morphology 10 Preparation of smear for staining 11 Procedure for identification of isolates Broth culture An agar slant culture An agar deep Terms to describe growth pattern 12 Motility test 13 Gram staining procedure 14 Sterilization Autoclave Hot air oven 16 Preparation of routine culture media Blood agar Chocolate agar Mac conkey agar Mannitol salt agar Dnase agar 17 2 S-S agar Tests for bacteria associated enzymes Catalase Coagulase Citrate ultilization Nitrate reduction Oxidase 20 Identification media TSI SIM MR-VP 26 Antibiotic sensitivity testing 30 Wet mount microscopy of intestinal parasites Concentration method Floatation Sedimentation 31 Examination of blood parasites Thick smear Thin smear 33 Staining of mycobacteria AFB staining Mycobacteria culture 34 An introduction to mycology practical Collection procedure Wet mount microscopy 35 Serological tests Technique of blood drawing Transfer of blood Factors causing of haemolysis Precautionary measures 37 RPR testing 39 Widal test 40 Some illustrations of pictures of laboratory concerns Microscope 41-52 3 Candle technique Anerobic jar Loop sterilization Haemolysis pattern in blood agar Blood agar and nutrient agar – staph. Aureus Mac conkey agar : lactose fermenter / nonfermenter Transfer of bacteria TSI interpretation SIM interpretation Eggs of intestinal helminthes Cestodes : egg and plroglottids Protozoal parasites (E. hystolytica & G lamblia ) Malaria parasites : Blood smear, P vivax, P malariae & P falciparum AFB staining procedure Fungus (Lactophenol cotton blue) Mucor, Penicillin & Aspergillus Fungus (Lactophenol cotton blue) Microsporum & Trichophyton There shall be lecture followed by demonstration and then the students will practice. Introduction The clinical microbiology laboratory of the hospital is more commonly known as a Diagnostic Laboratory of Infectious Diseases. There will be several sections or units such as Bacteriology, Parasitology, Mycology, Mycobacteriology, Serology / Immunology etc. The several categories of clinical microbiological specimens collected from the hospitalized patients as well as out patients services (OPD ) will be submitted in those different sections or units for laboratory diagnosis to help the clinicians for treatment management of the patients. These clinical materials always contain the pathogens; and therefore every one must follow : General rules / Guidelines of Microbiology laboratory: as a part of contamination / infection prevention General rules of microbiology laboratory 1. No eating or drinking in the microbiology laboratory. 2. Always disinfect the tables before and after lab. works 3. Wash your hands with soap both before and after lab, and in between the lab work. (alcoholic hand rub is also recommended) 4. Do not carry the nonessential materials (the only essential thing is the lab handouts and a notebook to write in). 5. Always use the proper aseptic technique when transferring cultures from one medium to another. 4 6. Heat the entire piece of wire loop in the flame: it should be red hot. Be sure to cool your inoculation instrument before picking up the colony or liquid broth. 7. Use an inoculating wire for agar deeps and an inoculating loop for the agar plate and the broths. 8. Label all test tubes and petri plates with your name (initials), your table #, date, exercise #, and name of organism. Do not use tongue to gum paper for label. 9. After finishing the lab work, discard the left over microbial cultures, test tubes in the rack, agar plate in autoclave bag on the autoclave cart for autoclave. 10. Do not dump ANY microbial suspension down the drain—only on the discard cart. 11. Place test tubes in racks when working at your table: never lay the tubes down—they leak. 12. Keep cover on the test tubes and petridish to reduce contamination it does not matter whether it is sterile media or already cultured 13. Always check agar plates carefully to make sure that there are no mold or bacterial contaminants on the plate. Do the same with any tube media that you pick up. if contaminated, discard the plate / tube on the discard cart. 14. All agar plates are incubated upside down before inoculation because It reduces bacterial contamination. Microscopy The microscope is absolutely essential to the microbiology laboratory. This is the first equipment of the laboratory. Therefore every student should know - the parts of the microscope and their functions. - how to use the microscope effectively, and particularly the oil immersion objective. This will be demonstrated and explained in the practical exercises Procedure to see the smear Place the prepared smear (or wet mount) on the stage, and secure it inside of the stage clips. 1. First use low power objective (10Xlens) Try to know where the specimen is located on the slide, and place it in the center of the hole allowing light through the stage; and then focus the smear 2. While looking through the ocular eyepiece, lower the stage slowly using the coarse adjustment knob. 3. As soon as you see the specimen, Stop using the coarse adjustment, and then use the fine adjustment knob 4. Locate good field for examination by oil immersion objective (100Xlens). 5 5. Now ready to move with oil immersion objective. Keep a small drop of microscopic oil. And then rotate the 100X objective (oil immersion) into place. The lens will touch to the oil drop. 6. Use fine adjustment to see the bacteria. Be sure that condenser is raised and light is adjusted. High power (40Xlens) is usually used for wet mount microscopy Note. a. Be sure to remove any oil by lens paper before covering the microscope. Do not use other tissue paper b. Once you have gone into oil immersion, do not go back to the 40X lens; oil will contact to the high power objective while changing the objective. The 10X can be returned if necessary, since the lens should not touch the slide anyway. c. Clean all lenses (oculars, objective lenses, and lens on condenser) with lens paper. One should not forget to clean the oil lens with lens paper. Laboratory growth media : Types of media: The types of media are described in following pages An agar medium: This contains agar (1.5-2%) as a solidifying agent; and this helps for isolation and purification e.g. Blood agar, Chocolate agar. MacConkey agar, Salmonella-Shigella agar etc. A broth medium: This has no agar; it is used for fast, luxuriant growth of bacteria e.g. Brain Heart Infusion broth (BHI broth) A semi-solid medium: This has a reduced percentage of agar, and can be used for motility e.g. SIM medium Microbiological specimens All the clinical specimens ( such as sputum, urine, pus swab, body fluid etc) must be proceeded soon after its reception. Delay causes the either overgrowth or pathogens may die because of drying of specimen. The specimen should be kept in the refrigerater particularly csf specimens because cold sensitive organisms die soon. 6 Specimen Rejection / Test Cancellation All specimens must be collected, labeled, transported, and processed according to standard laboratory procedure. Selecting the container type, volume, and proper handling of clinical material is essential for correct microbial diagnosis.. If the criteria for these processes are not met, the specimen may be rejected or the test may be canceled. The following represent some reasons for specimen rejection or test cancellation: • Insufficient volume for analysis • Improperly labeled specimen • Inappropriate specimen container • Improper specimen transport • Specimen that has leaked in transit • Specimen that has been sent in incorrect or expired transport media • Incomplete or incorrect test request form (e.g., testing not marked) • Test request form without a specimen • Specimen without a test request form • No specimen type, no source provided • Inappropriate specimen type (e.g. saliva in stead of expectorated sputum specimens). Specimen Labeling To assure positive identification and optimum integrity of patient specimens from the time of collection until testing is completed and results reported, all specimens submitted for testing must be adequately labeled. The label must have the patient’s name, age, sex with a identifying number (e.g., medical record number) and specimen type or source. Clients will be notified of inappropriately labeled specimens which will be returned to the client upon request Method of isolation from the clinical specimen : Streaking an agar plate 1. Gather all the necessary materials ( growth media, bunsen burner, transfer tools, etc). 2. Practice adjusting the flame of the bunsen burner. 5. Sterilize the loop or wire by holding in the flame of a gas burner. The metal must glow red before sterilization is considered complete. 6. Specimen is taken using sterile wire loop and streaked as demonstrated for the isolation of pathogen Routinely three kinds of isolation media are used in the microbiology laboratory. These are Blood agar, Chocolate agar and MacConkey agar. Until you become 7 well-acquainted with this procedure, you might want to draw the 3 sections that you will streak inside of, on the back (bottom of plate containing agar medium) with a sharpie pen. STREAKING AN AGAR PLATE Streaking Procedure: 1. Pick up a loopful of your inoculum from clinical specimen, a broth or an agar culture. 2. Using a sterile agar medium plate (lift the lid just enough to insert the loop), streak a vertical line straight down. 3. When streaking the agar, keep the loop horizontal and only streak the surface of the agar: do not dig into the agar. 4. Move the loop in a zig-zag pattern across the agar until 1/3 of the plate is covered, finishing the first section. 5. Sterilize the loop in the flame and let it cool before continuing to spread the bacteria. 6. You can do this by i) sticking the hot loop in the agar at the edge of the agar away from the bacteria, or ii) just holding the loop for a few seconds while it cools. 7. Rotate the plate about 90 degrees and spread the bacteria from the first streak into a second area using the same zig-zag spread technique. 8. Sterilize the loop again. Rotate the plate about 90 degrees and spread the bacteria from the second streak into the 3rd area in the same pattern. Sterilize the loop again. 9. Replace the lid and invert the plate. Incubate the plate. 8 All the plates, tubes or others if any are incubated aerobically in normal incubator at 36o C for 24-48 hours. You can see bacterial cells growing along streak lines and in isolated areas. Beside use of normal conventional incubator for normal incubation there are also necessary of other incubation environments such as reduced oxygen content and increased levels of carbon dioxide, anaerobic environment. Candle Jar technique : method to reduce oxygen content and elevate levels of carbon dioxide Purpose Certain specimens such as csf, sputum contain the pathogens that require reduced oxygen content and elevated levels of carbon dioxide during the incubation. The candle jar method creates an atmosphere with reduced oxygen and elevated levels of carbon dioxide. These conditions enhance the growth of microaerophiles. The candle used for this purpose normal plain candle (not colored candle). Principle The flame of the candle within a closed environment, will use up a certain percentage of the oxygen. When the available oxygen is reduced and elevated carbon dioxide created by the flame is increased, the flame will be extinguished. The plated medium within this atmosphere, will show enhanced growth of certain bacteria such as Haemophilus influenzae. The candle jar is incubated at 36o C as usual incubator. 9 Method of culture for anaerobes Anaerobic Jar (Brewer's Gas Pak or Mc Intosh Field Jar or BBL Jar) Purpose The anaerobic jar creates an artificial anaerobic environment (devoid of oxygen) which permits the growth of anaerobic bacteria. Principle The anaerobic jar employs a chemical reaction to generate hydrogen gas. In the presence of a palladium catalyst, the hydrogen gas will react with free oxygen in the air to form water. This reaction removes the oxygen from the sealed atmosphere. The jar is then incubated at the desired temperature. There will be slight negative pressure inside. Bacterial Colony Morphology Purpose Identifying and categorizing different, isolated bacterial colonial morphology (form and structure) will permit the selection and transfer of different species from a mixed culture, and allow transfer of a single colony to a sterile medium for cultivation of a pure culture. This exercise also shows how many diverse bacteria and fungi are present in our environment, as exhibited by the varied morphologies 10 Principles When a single bacterial cells is deposited on the surface of a nutritive medium, it begins to divide exponentially. After thousands (up to billions) of cells are formed, a visible mass appears. This mass of cells is called a colony. Therefore is defined as a visible mass of microorganisms all originating from a single mother cell, therefore a colony constitutes a clone of bacteria all genetically alike. Each species of bacterial or fungal organism will exhibit characteristic colonies Colony shape and size Margin (edge): Elevation: Color: Texture Colony characteristics (colonial morphology colony shape and size) are normally described as round, irregular, punctiform (tiny), margin (edge): entire (smooth), convex, umbonate, flat, raised, pigmented, opaque, translucent, shiny or dull: moist, mucoid, dry (or rough). Surface of colony:smooth, glistening, rough, dull (opposite of glistening), rugose (wrinkled) Consistency: butyrous (buttery), viscid (sticks to loop, hard to get off), brittle/friable (dry, breaks apart), mucoid Emulsifiability of colony: Is it easy or difficult to emulsify? Does it form a uniform suspension, a granular suspension, or does not emulsify at all? To determine emulsifiabiliti, the colony is suspended in a tube or water or saline solution to see how it goes into solution. Pick the colony with a loop, take it into the tube of liquid, and smear the inoculum against the glass wall right above the line of fluid within the tube. This ensures that the inoculum suspension becomes an even suspension rather than an undissolved mass of bacteria floating in the water. 11 Preparation of smear for Staining From an agar plate culture: Pick a bit of a colony from the plate showing the growth by use of flamed sterilized loop or straight wire. This is transferred to the slide containing a small drop of sterile normal saline and emulsify. A uniform thin smear is necessary. 1. The smear is thoroughly dried in air 2. The slide is then heat-fixed using one of the following techniques: a. by passing the slide through a flame to heat-fix the smear. b. use of a slide warmer can be used to heat-fix the slide. The heating adheres the smear to the glass surface and kills the cells Heat-fixing a bacterial smear to the slide by the use of the technique of passing the slide through a bunsen burner flame which can easily destroy bacterial cells if the slide is held in the flame longer than necessary. Inoculating the new medium for identification of isolates Procedure of taking the inoculum 1. Get the new medium into which which you are transferring the inoculum. Be sure that the new medium is labeled so you do not confuse the various cultures. 2. Heat the inoculating wire of the loop or needle until red-hot, and be sure that the entire wire is sterilized. You are now ready to pick the inoculum from the bacterial culture. A broth culture: The inoculums is transferred into the inner sides of the tube containing broth by use of bacteriological loop aseptically . An agar slant culture: Place the loop with the bacteria into the slant tube, all the way down to the bottom of the slant. There are 2 ways to inoculate the slant. If you want to identify the type of growth pattern, then just bring the loop straight up the slant. If you want to have a luxuriant culture, inoculate in a zig-zag pattern, starting at the bottom of the slant. This increases the surface area of the culture. An agar plate culture: as described on plate streak technique An agar deep: Use the needle to inoculate the deeps or semi-solid agars. Stab the inoculum down to the bottom of the deep in a clean, straight stroke. 12 Terms to describe growth patterns bacterial cultures in broth: Study of growth patterns in broth can be used for identification of organisms. These patterns can be characteristic for certain species. Remember, however, that these growth patterns can be influenced by growth conditions such as type of medium used or the temperature of incubation 1. uniform turbidity, or diffuse growth 2. flocculent (clumps) 3. sediment, and 4. ring or pellicle growth on surface Motility test: The motility of the organisms can be identified in different ways: The hanging drop wet mount and Semi-solid motility medium (SIM medium tube is also used for this purpose) 1. The hanging drop slide method : Use a small drop of bacterial suspension, but do not let it dry out (use a young culture of bacteria). Materials needed: i. fresh cultures (broth medium less that 24 hours old is optimal) of E. coli and either Bacillus or Pseudomonas ii. depression slides or coverslips and petroleum jelly Procedure: 1. Place a drop of the bacterial culture (optimally from a young broth culture) in the middle of a cover slip. 2. Place a thin line of petroleum jelly around the edge of the cover slide. 3. Turn the depression slide upside-down (depressed area facing down) and gently touch the cover slide. The jelly holds the cover slip to the slide and also keeps the suspension from drying out. 4. Now flip the entire microscope slide/cover slip combination over. It should look as shown in this figure 13 Looking at living bacteria are not as easy as one would think. First of all, living bacteria have no color, and they are small: therefore, they are really difficult to see, even with the oil immersion lens. Second, all bacteria have some vibrational movement, even nonmotile ones. This Brownian movement is caused by water molecules bouncing around in the solution, knocking up against each other and the microorganisms. Kinetic energy inherent to all molecules causes this kind of movement. On the other hand, those bacteria with flagella will be very apparently moving about the field of vision, although perhaps not all of the bacteria will be moving. Some cells will "run" straight across the field, others will "tumble" across the field in a slower motion. The medium is inoculated with an inoculating needle. The semisolid medium is stabbed with inoculum and then incubated. Next day we can see the result of the growth without the special stain and microscope. The movement of bacteria is detected along the line of inoculation Principle The lower agar concentration in the medium allows limited movement of motile bacteria from the area of the stab. Motility will be detectable as diffuse growth radiating from the stab line. A special dye, a tetrazolium salt (TTC) may be added to make the radiating growth more visible as the reddish diffuse area NOTE : The stab is performed with an inoculating needle. Care must be taken to insert the needle straight in and to pull it straight out. If the needle "slices" the agar horizontally, it may be difficult to tell if the resulting bacterial growth is from motile bacterial, or is just growth along a wide stab line. Motile bacteria move about with structures called flagella. Nonmotile bacteria without flagella are called atrichous. Categories of flagellation: monotrichous = single flagellum peritrichous = flagella all around lophotrichous = tuft of many flagella at one end or both ends 14 Gram staining procedure This staining is named after the Danish physician, Christian Gram, who developed this technique in 1884. It involves a series of simple steps. Gram staining method separates bacteria into two types. Gram-positive cells retain the crystal violet-iodine complex and thus appear purple Gram-negative cells are decolourised by the alcohol or acetone treatment, and then stained with safranin so they appear pink Gram-positive bacteria have a relatively thick wall composed of many layers of the polymer peptidoglycan (sometimes termed murein). The thickness of this wall blocks the escape of the crystal violet-iodine complex when the cells are washed with alcohol or acetone. Gram-negative bacteria have only a thin layer of peptidoglycan, surrounded by a thin outer membrane composed of lipopolysaccharide (LPS). The region between the peptidoglycan and LPS layers is termed the periplasmic space; it is a fluid or gel-like zone containing many enzymes and nutrient-carrier proteins. The crystal violet-iodine complex is easily lost through the LPS and thin peptidoglycan layer when the cells are treated with a solvent. Procedure: Make the bacterial smear from broth, slant, or plate as described earlier 1. If taken from a broth, use a loopful of the solution. 2. If taken from plate or slant, suspend the inoculum in a drop of normal saline on the slide and mix it well. 3. Spread the suspension on the slide so that it covers the size of one cm. circle 4. Place a piece of tape on the side of the smear so you know which way is up. 5. Air-dry and heat-fix the slide. 6. Place slide into container of crystal violet, leaving in for 1 minute. Wash well with water 7. Place slide into container of Gram's iodine, leaving in for 1 minute. Wash well with water. 8. Flood acetone-alcohol quickly on the slide, and wash off within 5-10 seconds (from beginning of decolorizer added). Wash well with water. 9. Place slide into container of safranin, leaving in for 1 minute. Wash well with water. 10. Blot dry with bibulous paper. 11. Focus on smear using low power lens, ending up on 100X oil immersion. Interpretation of microscopic finding: Gram positive bacteria will be blue/purple/violet. Gram negative bacteria will be light pink. Note: We have cultures of bacteria such as Staphylococcus, E. coli, or 15 Bacillus for doing Gram stain. We will give you gram + ve and Gram –ve bacteria to check your procedure Sterilization This is very important in microbiology laboratory. We do sterilization to destroy the bacteria and other microganisms. We do sterilization to growth media for use. We do sterilization for all the specimen collecting tools or containers. There are many methods of which autoclave and hot air oven are extensively used. In some cases we use disinfectant to kill the microorganism such as Glutaraldehyde, Hypochloride solution, Lysol disinfectant etc. Autoclave: This steam sterilizer is the most frequently used in the laboratory. You must know the details of this equipment Basically an autoclave is a huge steam cooker. Steam enters into a jacket surrounding the chamber. When the pressure from the steam is at a certain point in the jacket, a valve allows the steam to enter the chamber. The pressure will go up over 15 pounds per square inch (psi): at this point the timer begins to count down, usually for 15 minutes, depending on the type of media. The high pressure in a closed container allows the temperature to go above the highest temperature one could get by just boiling, around 121 degrees C. Therefore, the parameters for sterilization with an autoclave are 121o C at 15 lbs for 15 minutes. Fifteen minutes is the thermal death time for most organisms (except some really hardy sporeformers). 16 Hot Air oven Beside autoclave there is another very important equipment that is Hot air oven. This equipment Hot Air Oven is used for the sterilization of articles in dry condition. Hot air trapped after closing the door of the oven is heated to the 160 o C . This temperature is maintained for one hour (holding time). In this time period of 160o C ( the holding time) the articles will be free from the viable microorganisms including the spore formed organisms. The operation of Hot Air Oven will be demonstrated. Preparation of Routine Microbiology Media Normally there are two type of media : nutrient broth and nutrient agar. These two media; one is a liquid and the other is a solid. The solid media is prepared by addition of agar agar (a sea weeds), an extract from the cell walls of red algae. These days all the laboratory growth media are easily available in the dehydrated form in the market. We simply need to rehydrate the powder form of the medium as described in the instruction procedure contained in the purchased dehydrated media; and all these prepared media are autoclaved. 17 The laboratory staff will demonstrate making of NA (Nutrient agar) plates, NA slants, and Nutrient broth The procedure: The flasks of melted nutrient agar are kept in the water bath which is set to 45oC. This is to prevent them from solidifying, since agar solidifies at around 42oC. The flask will be taken from the bath only when ready to pour the plates. Set the petri dishes out (small side down), tops covering the dishes. When it will be ready to pour the plates, the staff will take the flask of agar and pour the plates normally 20 ml per petri dish. Cover the bottom of each plate, Allow all plates to stand until they are completely solidified. Once they have solidified, place them on the tray upside down (bottom dish with agar on top). Why are agar plates incubated upside down? Two reasons: There may be air contaminants in the incubator, it will be more difficult for them to get into the plates. Often we will see a bit of water condensation on the top of the agar plate, particularly when they have been at 37 oC. The water molecules are cohesive and tend to run together. If the plate was sitting right side up, the water droplets could then fall onto the agar, creating a kind of little lake on the agar plate and messing up your plates. Upside down plates prevents the condensation from dropping on the agar surface. Routine used media Blood Agar Blood agar is used both as an enriched medium for growing fastidious bacteria and as a differential medium to study the pattern of haemolysis. The hemolysis pattern is an especially useful tool for identification of many of the Gram positive cocci. Hemolysins are grouped in three categories: 1. Beta hemolysins completely lyse the red blood cells and hemoglobin; this results in complete clearing around colonies. 2. Alpha hemolysis refers to the partial lysis of RBC's and hemoglobin and produces a greenish discoloration of the blood agar around the colonies. 3. Gamma hemolysis, sometimes called NO hemolysis results in no change of the medium. When reading plates for type of hemolysis, be sure to look for color changes in the surrounding agar. A common mistake is to look at the color of the bacteria, rather than the agar. MacConkey Agar 18 MacConkey agar is a widely-used culture medium which is both selective and differential. The medium is primarily used to differentiate between Gram negative bacteria while inhibiting the growth of most Gram positive bacteria. The medium also differentiates between lactose-fermenting coliforms and non-lactose fermenters, which include potential pathogens This medium contains bile salts and crystal violet that will inhibit the growth of most Gram positive bacteria, making MacConkey agar selective. Lactose, a fermentable carbohydrate, and neutral red, a pH indicator, are added to differentiate the lactose fermentors from the pathogenic lactose nonfermenters. When lactose is fermented, acid products lower the pH below 6.8, with the resulting colonial growth turning pinkish-red. If an organism is unable to ferment lactose, the colonies will be colorless. MacConkey is selective: Gram negative organisms will grow, Gram positive organisms will not. This is due to the addition of bile salts and crystal violet. MacConkey is differential: Lactose fermenters will appear pink, non-lactose fermenters will appear colorless. This is due to the addition of the indicator, neutral red. Mannitol Salt Agar Staphylococcus is the most commonest isolates’; therefore this medium is used in the laboratory. Mannitol salt agar is both a selective and differential growth medium. This medium helps determine two characteristics of bacteria, whether they are salt tolerant or not, and whether they are able to ferment mannitol or not 1. Salt Tolerance : This medium contains 7.5% salt higher than normal mediuim ( about 0.5% NaCl in the routine isolation medium) and therefore "selects" for organisms that are able to tolerate the presence of high levels of salt. If the organism grows, it is salt tolerant; if no growth it is not salt tolerant. 2. Fermentation of mannitol. This medium contains an indicator, phenol red. The indicator is a pinkish-red at neutral pH, is really red at pH 7.4 or above and is yellow below pH 6.8 (acidic). An organism which can ferment (metabolize) mannitol will be indicated by a color change on the MSA plate(which makes this a differential test; and if no fermentation the medium will remain red (no change). If the organism does ferment mannitol, it will create metabolic by-products which are acidic--and the surrounding medium will be yellow. DNase Test DNase test is used to identify bacteria capable of producing the exoenzyme DNase This enzyme catalyzes the depolymerization of DNA into smaller 19 fragments is called a deoxyribonuclease, or DNase. The test agar contains an emulsion of DNA, peptides as a nutrient source, and methyl green dye. The dye and polymerized DNA form a complex that gives the agar a blue-green color at pH 7.5 . Bacterial colonies that secrete DNase will hydrolyze the DNA in the medium. This results in clearing around the bacterial growth Salmonella Shigella agar (SS agar) This is the selective media for isolation of Salmonella and Shigella organism. In this media all coliform bacilli group of bacteria are inhibited. However certain strain of coliform bacteria can grow The stool culture findings are usually positive in approximately 85-90% of patients with typhoid fever during the first week, declining to 20-30% later in the course of the disease. The conventional culture techniques usually take 48-72 hours from acquisition until the organism is identified. The bacterial dysentery caused by Shigella species are also isolated by this medium The stool specimen is collected in a wide mouth sterile (preferably) wide mouth container. One loopful of mucus portion preferably blood containing material is and inoculated into SS Agar (DCA Dexycholate agar is also used in parallel in many countries. A single rectal swab culture at hospital admission can be expected to detect S typhi in 30-40% of patients. The plates are incubated overnight at 37 oC. Salmonella and Shigella bacteria are non lactose fermenter.. The isolates are further identified by motility test and biochemical tests The biochemical tests includes TSI, SIM, Citrate, MR-VP Serology of isolate is done for species identification. Tests for bacteria associated enzymes Catalase test : catalase is an enzyme that splits hydrogen peroxide into water and oxygen. Hydrogen peroxide is a by-product of respiration and is lethal if it accumulates in the cell. Catalase is an enzyme that can degrade the hydrogen 20 peroxide in the cell before it can do any cell damage. It splits the H2O2 to free oxygen (bubbles) and water. Important point : Use growing bacterial colony (plate or tube) You can drop 3% H2O2 directly on an agar plate / slant, but it may kill the culture. The procedure: Slide method Pick the inoculum from a plate culture or slant culture and place it on a slide. Add one drop of 3% H2O2. Result: You will see a reaction if the test is positive, most often lots of bubbles. Slight bubbles also indicate a positive reaction. CATALASE TEST Coagulase test This is one of the most common test Why we do this test? To differentiate potentially pathogenic Staphylococcus species from other Gram positive and catalase-positive cocci. It is thought that an infective organism that produces the coagulase enzyme may protect itself by inducing clotting in surrounding tissues, thereby inhibiting destruction by normal body defense such as phagocytosis or antibodies 21 Purpose This test is used to detect the ability of certain Staphylococcus species to clot plasma. Coagulase production is a characteristic of the potentially pathogenic S. aureus. There are 2 methods (slide test and tube test) : Both tests use the same substrate; The tube test is more accurate, but the slide test is faster. Material needed: 0.5 ml rabbit plasma per test Slide agglutination test Make a 2 cms diameter circle on a clean glass slide using a wax pencil. Place two drops of thawed rabbit plasma into the circle, using a wooden pick or a clean loop. Add a single colony and emulsify it in the plasma. Add a single colony and emulsify it in the plasma. Fibrin threads form between the cells, causing them to agglutinate, or clump. There will a visible clumping of cells within 10-15 seconds. This test is for the bound coagulase enzyme. Tube agglutination test Inoculate a tube with a ½ ml of rabbit plasma with the bacterial inoculum. Place at 37C and check at ½ hour and after (some strains will give a + reaction in a few hours, other strains take longer) by tipping the slide at an angle. Any degree of coagulation is considered a positive test for the free coagulase enzyme. Add a single colony and emulsify it in the plasma. Fibrin threads form between the cells, causing them to agglutinate, or clump. There will a visible clumping of cells within 10-15 seconds. This test is for the bound coagulase enzyme. Rabbit plasma is inoculated with the organism 22 Citrate Utilization Test Purpose The citrate utilization test is used to determine the ability of an organism, using the enzyme citrase, to use citrate as its sole carbon source. The citrate test identifies the use of citrate as a sole carbon source, since there are no other nutrients in this medium. The basic end products will cause the brom thymol blue indicator in the medium to turn from forest green to royal blue. Principle Simmon's citrate agar is a medium containing sodium citrate as the sole carbon source and the ammonium ion as the sole nitrogen source. The pH indicator, bromthymol blue, will turn from green at neutral pH (6.9) to blue when a pH higher than 7.6 is reached (basic or alkaline). If the citrate is utilized, the resulting growth will produce alkaline products (pH >7.6), changing the color of the medium from green to blue. In this medium, sodium citrate is the sole source of carbon and energy. This test determines whether or not an organism is able to metabolize citrate for energy. The uninoculated medium is green. An indicator, bromthymol blue, is added to the medium, which changes color based on pH. The citrate will be yellow if the metabolic products are acidic. A color change to royal blue indicates alkaline byproducts. Any color change (from green to either yellow or blue) represents a positive test for citrate utilization. Nitrate Reduction Test Purpose This test detects the ability of an organism to reduce nitrate (NO 3) to nitrite (NO2) or some other nitrogenous compound, such as molecular nitrogen (N2), using the enzyme nitrate reductase Principle Nitrate (NO3) may be reduced to several different compounds, either by anaerobic respiration or by denitrification. This test is used to detect whether or not the reduction has taken place. The nitrate medium contains potassium nitrate 23 as the substrate. If the organism reduces the nitrate to nitrite, the nitrite will react with added reagents sulfanilic acid and a-naphthylamine to produce a red color. If no color is produced, this can indicate either of two reactions: (1) the nitrate was not reduced (2) the nitrate was reduced even further to compounds other than nitrite. To distinguish between the negative reaction, or the complete reduction, zinc dust is added. If nitrate remains in the medium, zinc will reduce it to nitrate, and a pink color is observed. This is a negative reaction. No color change after zinc is added means that nitrate has been reduced to compounds other than nitrite. This is interpreted as positive and is often call positive complete to distinguish it from the first positive test discussed Reagents : Nitrate broth nitrate reagents A (sulfanilic acid) and B (naphthylamine) wooden sticks for zinc zinc powder Procedure: Inoculate the nitrate broths with your bacterial unknown. Incubate at the optimal temperature, 30 or 37C, for your organisms. Result: After incubation: Look for N2 gas first before adding reagents; and then add 6-8 drops of nitrite reagent A. After this add the same number of drops of nitrite reagent B. One can see a reaction within a minute or less. If you have not seen either nitrite or N2 gas, you need to add a bit of powdered zinc. A bit of zinc is about the amount that sticks to the end of a wood stick. Result Reaction N2 gas NO3 to NO2 NO3 to NO2 NO3 to ammonia NO3 – no reaction none yes None None Color after adding reagent red no color no color no color Color after adding zinc Not added no color no color pink color Nitrate Test Reagents Reagent A: Sulfanilic Acid Reagent B: alpha-naphthylamine 24 Zinc dust is added to tubes that appear to be negative (remain clear) after the addition of Nitrate Reagents A & B Oxidase test This test is used to identify bacteria containing the respiratory enzyme cytochrome oxidase.The oxidase test is a key test to differentiate between the families of Pseudomonadaceae (Oxidase positive) and Enterobacteriaceae (Oxidase negative). The enzyme cytochrome oxidase is involved with the reduction of oxygen at the end of the electron transport chain. The colorless reagent used in the test will detect the presence of the enzyme oxidase and, reacting with oxygen, turn a color. Principle The cytochrome oxidase enzyme catalyzes the transport of electrons from a donor compound to the final electron acceptor, oxygen. In this test, an artificial electron donor, tetramethyl-p-phenylenediamine, a redox dye in its reduced form, is used to reduce the cytochrome oxidase. If the enzyme is present, the colorless dye will turn a purple to blue color. No color change is a negative test. A couple of key points when doing this test: We keep the oxidase reagent either frozen or unopened in tubes until needed. If old reagent is sitting out on the bench and is purple, ask for a new tube from the instructor Use a young culture, preferably less than 24 hrs old. Use fresh reagent, less than a couple of hours old (it is taken out of the freezer). Pick your inoculum, not with a metal loop (reagent may react with the metal), but with a wooden stick. Read the reaction within 20 seconds (not after), usually it will change in less than 15 seconds. The oxygen will change the reagent color as time passes, so it must be read quickly. 25 Materials needed: oxidase reagent (Tetramethyl-p-phenylenediamine) wooden rods Procedcure: Pick a good-sized amount of inoculum from a plate culture or slant culture and place it on a piece of filter paper. Add one drop of the reagent (if it is dark blue, it is old and should not be used). OR you can drop the reagent directly onto the slant or plate, but that might damage your culture. Result A positive reaction, bluish-purple will occur within 20 seconds. color that progressively becomes more purple Do not read the reaction after 30 seconds. Decarboxylase Test This test is used to detect the ability of an organism to decarboxylate an amino acid. Decarboxylation is a reaction which removes the carboxyl group (COOH) of an amino acid, producing an amine and carbon dioxide. The amino acid is added to the test medium, along with the pH indicator, bromcresol purple, and the medium is sealed with mineral oil after inoculation. This creates anaerobic conditions which promote fermentation. Accumulation of acid end products from fermentation is necessary because decarboxylase enzymes are inducible only in the presence of substrate and acid environment. The decarboxylation of the amino acid by the enzyme then results in alkaline end products. These in turn will cause the pH indicator to turn purple (positive). Lysine and ornithine are the amino acids which are tested for decarboxylation. Arginine, another amino acid tested, is a different chemical reaction: dihydroxylation. However, the test results are interpreted in the same way. Identification media After the isolation of pathogens it must be identified by use of idendtification media as well as the biochemical tests Triple sugar iron agar (TSI) This is a stab and streak inoculation technique. This is a good medium for culture of bacteria. This medium is used for Gram negative rods usually . TSI agar tests for knowing three things : They are sugar fermentation (glucose/lactose/sucrose), CO2, and H2S. Carbon dioxide is identified by cracks and bubbles inside of the medium, sometimes a few bubbles and sometimes enough to push the slant up to the top. 26 TSI agar contains peptone, glucose, sucrose, lactose, and thiosulfate. Phenol red is the pH indicator (yellow at pH less than 6.8 and red above 6.8). It is prepared on an agar slant, with a deep butt to provide for anaerobic growth. Procedure Inoculate the medium using an inoculating wire. Stab the inoculum down through the butt, then pull the needle out and streak up the slant ( Do not take another inoculum to do the slant). Incubate at 37 o C. There will be demostration so that you can see some different reactions using this medium. There are 3 possibilities of sugar reactions and different reactions in different areas (butt vs. slant) of the medium, so the physiology behind it is pretty complex. The outcome of sugar use is always acid, so the pH indicator phenol red will turn yellow--reported as A. No use of the sugar or alkaline by-products (which is NO sugar use) from the other non-sugar nutrients in the medium will cause the indicator to stay the same color red/orange or maybe even change it to a red---reported as a K. The reactions in TSIA are reported as slant (A or K), butt (A or K), a circle around the butt for CO2, and + for H2S. For example K/A +H2S = red slant, yellow butt, with both CO2, and H2S. The glucose (=dextrose) is 1/10 in concentration as the other 2 sugars. The fermentation of the sugars causes the anaerobic butt to turn yellow and stay yellow. However, if only glucose is used, even though the slant turns yellow only after a few hours it will revert to red because the protein in the medium is broken down to alkaline products when the small amount of glucose is used up. If lactose and/or sucrose are used, the large amount of fermentation products neutralizes the basic products and the slant stays yellow. The following symbol is used to express the result A/A = glucose and lactose and/or sucrose are used K/A = glucose alone K/K = no sugars used NOTE: Purpose of TSI test is to differentiate bacteria based on their ability to ferment glucose, lactose and/or sucrose, and to reduce sulfur to hydrogen sulfide. It is used primarily to distinguish the morphologically similar bacteria of Enterobacteriaceae, all of which ferment glucose to an acid end product. There is no way to get a A/K reaction when using a Gram negative rods on this medium. IF you do, it means that 1) you did not inoculate correctly with a stab and streak or 2) you inoculated something other than a Gram negative rods. 27 SOME COMMON SUGAR REACTIONS IN TSI Slant /butt Symbol Interpretation Red / Yellow Yellow / yellow K/A A/A Red / Red K/K Red / no color change K/NC Yellow / yellow with gas A/A, G Red / yellow with Gas And black precipitate Yellow / Red And black precipitate Yellow / yellow And black precipitate No change / No change K/A,G, H2S Glucose fermentation, Glucose & Lactose and or Sucrose fermented No fermentation Peptone catabolised No fermentation Peptone used aerobically Glucose & Lactose and or Sucrose fermented with Gas Glucose fermented and gas H2S produced Glucose fermented only H2S produced Glucose, Lactose and or Sucrosed fermented, H2S No fermentation K/A, H2S A/A, H2S NC/NC A = Acid production;K = Alkaline reaction H2S = Sulphur reduction G = Gas Interpretation: A/A = yellow throughout e.g. Esch.coli K/A = red slant, yellow butt e.g. Salmonella, Shigella K/K = red or red throughout e.g. Pseudomonas carbon dioxide = bubbles or breaks in medium black precipitate = hydrogen sulfide SIM Medium This medium is used for three tests: they are Sulphur Reduction test, Indole Production test, Motility test. This is particularly important in differentiating certain enteric organisms Sulphur Reducton Hydrogen sulfide, H2S, can be formed by putrefaction or anaerobic respiration. The medium contains cysteine, an amino acid containing sulfur, and sodium thiosulfate plus peptonized iron or ferrous sulfate. The H2S will react with the iron or ferrous sulfate, forming a black precipitate. If the black precipitate is present, the test is positive for H2S production. No precipitate is a negative test 28 Test for Indole Production The indole test is used to identify bacteria capable of producing indole using the enzyme tryptophanase. The enzyme tryptophanase can convert the amino acid, tryptophan, to indole, ammonia, and pyruvic acid. The by-product, indole, is the metabolite identified by this test. When Kovac's reagent, which contains hydrochloric acid and dimethylaminobenzaldehyde and amyl alcohol, a red layer will from when indole is present. No color in this layer is a negative result. Motility test from semisolid agar This medium can be stab-inoculated with an inoculating needle to indicate motility. The lower agar concentration in the medium allows limited movement of motile bacteria from the area of the stab. Motility will be detectable as diffuse growth radiating from the stab line. Methyl red (MR) and Voges-Proskauer (VP) tests The MR - VP tests are run together in the same broth and then split into 2 tubes when ready to be tested for the end products. The methyl red test determines the use of glucose, with the subsequent production of acid, tested for by the pH indicator methyl red. The VogesProskauer test also determines glucose use, but for a different end product---not acid but a neutral product called acetoin (or acetylmethylcarbinol). The VP is really important for identification of many bacteria, and must be done carefully. Determine the various reactions for these media: MRVP broth, . 1 MRVP broth per unknown Barritt's reagents A (alpha-naphthol) and B (KOH) methyl red reagent The procedure: Inoculate into the MRVP broth. Incubate at 25 or 37 degrees C. AFTER INCUBATION: Pour 1/3 of the suspension into a clean nonsterile tube: run the MR test in the tube with 2/3, and the VP test in the open tube with 1/3. for methyl red: Add 6-8 drops of methyl red reagent. 29 for Voges-Proskauer: Add 12 drops of Barritt's A, mix, 4 drops of Barritt's B, mix. Let sit, undisturbed, for at least 20 minutes. Interpretation: Methyl red Within just a few seconds after adding methyl red reagent, you can see the red-pink color of acid presence from glucose use.. Voges-Proskauer tests The reagents MUST be added in the correct order, in the correct amounts, and the tube must sit undisturbed, and open to the air (no cap) for at least 30 minutes (45 minutes is even better) as the light pink color intensifies at the top of the tube (the reagents react with acetoin). Do not shake the tube after sitting it down for the waiting period. Antibiotic Sensitivity Testing Antibiotic sensitivity testing is done to determine the susceptibility of bacteria to various antibiotics. This standardized test is used to measure the effectiveness of a variety of antibiotics on a specific organism in order to prescribe the most suitable antibiotic therapy There are several methods of doing the antimicrobial susceptibility testing. Of those different method Disc Diffusion Technique is one widely followed in all clinical microbiology laboratories of the globe. This technique is now well standardized and recommended by NCCLS (National Committee of Clinical Laboratory Standard) U S A. A series of antibiotic-impregnated paper disks are placed on a plate inoculated to form a bacterial lawn (even, confluent growth). The plates are incubated to allow growth of the bacteria and time for the antibiotics to diffuse into the agar. If an organism is susceptible to an antibiotic, a clear zone will appear around the disk where the growth has been inhibited. The size of this zone of inhibition depends on the sensitivity of the bacteria to the specific antibiotic and the antibiotic's ability to diffuse through the agar The Kirby-Bauer test must be carefully standarized. This means that a special agar, Mueller-Hinton agar, is used along with a prescribed inoculum of broth. The antibiotic disks are also standardized to contain a specific amount of antibiotic. After 18 hours of incubation at 35oC, the clear zones are measured. These are compared with tables giving the interpretation of measurement for each antibiotic. In order to determine if an antibiotic will be effective in treating the bacterial infection, the zone of inhibition must be measured and compared to a standard chart provided by NCCLS, USA. Compare the zone of inhibition of test result with 30 the Standard Chart and issue result as Sensitive, Partial sensitive or Resistant . An organism is not considered to be sensitive to an antibiotic unless the zone of no growth is matched with NCCLS Chart The sensitivity test result Reading & reporting Wet mount microscopy of Intestinal parasites Wet mount microscopy: Procedure 1. A drop of normal saline (0.9% Sodium Chloride) is kept on a new slide. 2. A small amount of stool specimen is taken by use of wooden applicator 3. This stool is emulsified with the normal saline. 4. This mixed suspension should not very thick 5. A cover glass is kept over this mixed suspension 6. It is examined by low power objective of the microscope (X100) and checked if necessary by high power objective (x 400) 7. Examination must be completed before drying of the preparation. 31 Concentration method / technique: This method is used when the ova, cysts, larvae are in scant numbers Types of Concentration method 1. Flotation method uses the high specific gravity of a solution to float the lighter ova and cysts a. Make saturated solution of Sodium chloride b. Keep a little quantity amount 0.5 – 1ml into the penicillin vial or the tube that can hold coverglass. c. Keep stool sample about 3-4 times of matchstick head and emulsify with the saturated solution of Sodium chloride d. After smooth emulfication keep the saturated NaCl and mix it; continue mixing this way to the neck of the vial of tube. The tube must be in vertical position. e. Keep cover glass in touch with the solution and wait for 30 minutes. 2. Sedimentation method : Modified Ridley-Allen method for sedimentation technique.This technique uses formalin as a preservative and ether or ethyl acetate as an extractor of fat and debris from faeces. Note: Ether is flammable and formalin is an irritant Materials. a. Formalin water (100ml formaldehyde and 900ml distilled water) b. Ether or ethyl acetate. c. Mesh (425µm) brass wire filter, 3 inches in diameter (Endcott Sieves Ltd. Lombard Road, London SW19 3BR). If this is not available ordinary nylon tea strainers provides an alternative substitute (This is of low cost) d. Small 3inches porcelain or stainless steel dish. Method. a. Using swab sticks, select a quantity of faeces (approximately 1g or pea size) to include external and internal portions. b. Place the faeces in a centrifuge tube containing 7 ml of 10% formalin. c. Emulsify the faeces in the formalin and filter through the brass/nylon filter into the dish. This filtration process allow the parasites to go through the solution. d. Wash the filter and discard any lumpy residue. e. Transfer the filtrate to a tube and add 3 ml of ether/ethyl acetate. Mix well on a vortex mixer for 15 seconds or by hand for 1 minute. 32 f. Transfer back to the centrifuge tube and centrifuge at 3,000 rpm. for 2 minute. g. Loosen the fatty plug with an orange stick and pour the supernatant away by quickly inverting the tube. h. Allow the fluid on the side of the tube to drain on to the deposit and mix well. Transfer a drop to a slide for examination under a coverslip. i. Use the x10 and x40 objectives to examine the whole of the deposit for ova and cysts. Note: The important points to be considered when performing a concentration technique are: 1. In a specimen the whole of the sample (equivalent to 1 gram of faeces) should be concentrated and the whole of the deposit examined. This corresponds to good practice with clinical samples. 2. It is important to vortex the sample for at least 15 seconds after the addition of ether or ethyl acetate, as failure to do so may result in excess deposit, thus obscuring ova and cysts. 3. Adequate centrifugal force must be used because if this is below the required value, there may be insufficient gravitational force to sediment the ova and cysts. The centrifugal time is also critical, since the ova and cysts may remain in suspension if the sample is not centrifuged for the minimum required time. It is recommended that the sample be centrifuged at 1000g for 2 minute. Examination of Blood Parasites Preparation of thick and thin blood films :Thick films:- place a drop of blood in the middle of a clean microscope slide and with the corner of a second slide spread the drop until it is about the size of a five cent coin. The thickness should be such that it is just possible to see news print through it. Thin film :- Thin film are made as demonstrated ( also shown in the figure). Allow the films to dry, do not leave on the bench in a laboratory which is not fly proofed otherwise the film will be eaten When the films are dry, fix and stain the films by any method of Romanosky stain such as Giemsa stain, Field’s stain, Wright’s stain, Leishman stain etc but remember the pH of the stain should be a slightly alkaline (pH 7.2) as an acid stain may fail to show the parasites. 33 The films are stained by Giemsa stain : Giemsa is diluted (1/20) and keep in the staining jar so that the film is in an upright position, this will allow any debris to fall to the bottom of the jar. Stain for about 30 minutes, wash gently with clean water and allow to dry. If available use a positive control. Field's stain is also used because it is very quick. Field's stain comprises two solutions; a polychrome methylene blue (A) and eosin (B). The solutions are kept in covered staining jars. Dip the dry but unfixed film into solution A for 1 or 2 seconds. Remove from solution A and immediately rinse in clean water ( a 250ml beaker with water gently flowing into it is suitable) Dip the film into solution B for 1 or 2 seconds. Rinse in clean water for a few seconds. Place in a vertical position to dry. Under the microscope examine the stained slide using an oil immersion Staining for Mycobacteria (Demonstration of AFB and Practice of AFB staining) AFB staining procedure ( Also known as Ziehl Nelson’ stain): The term AFB 'acid fast bacilli' refers to an organism's ability to retain the carbolfushcin stain despite subsequent treatment with an ethanol-hydrochloric acid mixture. The high lipid content (approximately 60%) of their cell wall makes mycobacteria acid-fast. 1. Flame slides to heat fix 2. Flood the entire slide with Carbol Fuchsin 3. Ensure enough stain is added to keep the slides covered throughout the entire staining step. 4. Using a Bunsen burner, heat the slides slowly until they are steaming. Maintain steaming for 5 minutes by using low or intermittent heat (i.e. by occasionally passing the flame from the Bunsen burner over the slides) 5. Rinse the slide thoroughly with water 6. Flood the slide with 3% acid-alcohol and allow to decolorize for 5 minutes.Throughout the 5 minutes, continue to flood the slides with 3% acid-alcohol until the slides are clear of stain visible to the naked eye. 7. Rinse the slide thoroughly with water and then drain any excess from the slides. 8. Flood the slide with the counterstain, Methylene Blue. Keep the counterstain on the slides for 1 minute. 9. Rinse the slide thoroughly with water .Result : AFB – Red color bacilli; and Pus cells and other materials - Blue 34 AFB Staining : China modified method 1. A smear is made, dried and fixed by heat (flaming) 2. Flood the entire smear with dye # 1 for 3-5 minutes. 3. Rinse the slide with water 4. Flood the slide with dye #2 for one minute 5. Rinse the slide with water 6. Dry it and look by microscope using oil immersion objective Note: Dye #1 Basic red 4g Carbolic acid 8g 90% Ethanol 20 ml Water 100ml Dye#2 Methylene blue 1g Conc. Sulphuric acid 20ml Ethanol 30ml Water 50ml Mycobacteria culture Culture : a brief introduction Lowenstein-Jensen medium (For culture of Mycobacterium tuberculosis) After digestion and concentration by treatment with sodium hydrochloride solution, the mucopurulent sputum material is cultured on this selective media, and then incubated for up to 8 weeks at 36o C. Result : The Positive growth appears pigmented (Yellow pigment) which is rough and hard to remove from the growth medium Culture of AFB is carried out in safety cabinet because the bacteria can remain viable for several days or week; and easily transmitted to the staffs. An introduction to Mycology practical Dermatophytes are fungi that can cause infections of the skin, hair, and nails. The organisms colonize the keratin tissues and utilize keratin. These infections are also known as ringworm or tinea. Some time the organisms do invade the subcutaneous tissues There are three genera of dermatophytes, Trichophyton Microsporum Epidermophyton 35 Specimen collection : Skin, nail or hair scrapings or cutting collected in nonsticky glazed paper or sterile container e.g. petriplate in case of skin and nail Collection Procedure : Skin, nails and hair Cleanse the area with 70% alcohol prior to specimen collection. Skin should be taken from the active border of the lesion Nail should be from a subsurface portion of the infected nail. scrapings or cutting collected in nonsticky glazed paper or sterile container e.g. petriplate in case of skin and nail. Hair should be plucked, not cut, from the edge of lesion. Take 10-12 hairs. Specify the source of the specimen and include any pertinent clinical information. Wet mount microscopy (Direct Examination) • A small sample of the specimen is selected for direct microscopic examination and investigated for the presence of fungal elements. • The specimen is mounted in a small amount of potassium hydroxide or calcofluor white. • The KOH slides are gently heated and allowed to clear for 30 to 60 minutes before examining on a light or phase contrast microscope. Note : 10% KOH is for skin scraped material 15% KOH is for nail pieces 20% KOH is for hair pieces Result When present in the direct examination dermatophytes appear as hyaline (nonpigmented), septated elements. Fungal hyphae appears as branched filaments making up a mycelium When hair is involved the arthroconidia may be found on the periphery of the hair shaft (ectothrix) or within the shaft (endothrix) Cultures are incubated up to 3 weeks before a final report is issued. The cultures are incubated at 30°C and examined frequently for 4 weeks. If the dimorphic fungi is suspected the there should be incubation at 37o C also. 36 Dermatophytosis Skin KOH Serological test Introduction Serological tests are done to demonstrate either antigens or antibodies in the serum. The VDRL / RPR / TPHA / HIV testing are the serological tests. Usually the specific antibody is detected in the serological testing. Enzyme linked immunosorbent assay (ELISA) is one method extensively followed in many laboratories. In ELISA technique the microplate in which the wells are coated with the specific antigens is used. Technique of drawing the blood: 1. The patient is seated and the fore arm is stretched on the table with supporting cushion if necessary 2. Using the tourniquet on the upper arm the patient is told to clench the fist. 37 3. The median cubital vein is identified 4. Clean the area with 75% alcohol swab.(Ethanol / Isopropanol) 5. The vein is palpated if required 6. Insert the needle with bevel side up and parallel to the vein 7. Withdraw the 5ml. of blood at least 8. Tourniquet is released and the patient is told to open the hand Alcohol swab is applied and pressed for some time or Adhesive tape is applied. Transfer of blood: 1. Blood is transferred slowly into the well cleaned and dried tube which is already labeled with patient identity. 2. The needle is destroyed with use of needle destroyer and then discard into the puncture proof container. 3. The tube is centrifuged at slow speed (1500-2000rpm) for 5 minutes 4. The separated serum is transferred into a cleaned tube for RPR, TPHA and HIV testing as per necessary; and the remaining serum is transferred into another sterile plastic vials( e.g. cryotubes / eppendorf tubes) for storage at -20oC labeled with patient’s identity number The factors causing of haemolysis (Haemolysed blood is not suitable for serological test) Haemolysis often occurs in 1. blood taking by too small bore of a needle 2. expelling blood quickly from syringe 3. forced suction of blood in the syringe during blood collection. 4. vigorous shaking of blood 5. centrifuging blood sample at a high speed before clotting 6. freezing and thawing of blood 7. unclean tubes with residual detergents water in the tube Precautionary measures in practical of blood specimens 1. Avoid skin contact with blood. 2. Any blood that comes in contact with floor must be decontaminated with hypo chlorite 3. All the other details of the laboratory procedures will be described in this procedure manual in the following chapter. 4. Avoid recapping needles because there is high risk of infection through needle stick injuries. If recapping is necessary, recap using only one hand (i.e. by placing the cap on the table and inserting the needle inside the cap) 38 Rapid Plasma Reagin (RPR )Test Introduction The RPR test is the primary screening test for syphilis antibodies in serum. This is non-treponemal flocculation test . Treponema pallidum, a causative organism of Syphilis, produces antibody in response to tissue damage. This test if positive the titration is required. The titration indicate the possibility of current activity and post treatment. The RPR positive test is confirmed by TPHA. In RPR technology the antibody is detected by cardiolipin antigen; and therefore this antibody also known as cardiolipin antibody. In the earlier years Wasserman reaction test was done for sorodiagnosis of Syphilis; later on VDRL test came in the market; now RPR test is followed by many laboratories because - it is very easy to perform - it is not necessary to heat serum - this test can be done even by plasma. Before testing the entire reagent kept in refrigerator (2 –8o C) is taken and allowed to stand outside to acquire room temperature. Test Procedure 1. With use of a clean and dried Pasteur pipette with rubber teat or disposable plastic dropper dispense one drop of serum on to a circle of the test card and spread it using an applicator stick. 2. Keep one drop of antigen suspension using a dispensing dropper (provided in the test kit) into the test card 3. Rotate this test card at 100 rpm for 8 minutes keeping on an rotator 4. As a part of quality control every test run should include a control serum Interpretation of results : Medium and large aggregates Finely dispersed aggregates No aggregate (i.e. smooth appearance) Reactive weakly reactive No reactive Note: False positive reaction may occur in diseases for example Tuberculosis, Leprosy, Malaria, Infectious mononucleosis, Cancer etc. Because of this reason a specific treponemal test should be performed i.e. TPHA for the detection of specific Antitreponemal antibodies. 39 Widal test This is a serological test; method of doing varies depending on the manufactures. Therefore manufacture’s testing kit procedure (leaflet) is to followed. The principle of the test is similar to RPR testing. The Widal test is the traditional serologic test used for the diagnosis of typhoid fever. The test measures agglutinating antibodies against flagellar (H) and somatic (O) antigens of S typhi. In acute infection, O antibody appears first, rising progressively and later on falls often disappearing within a few months. High O antibody titers generally indicate acute infection. Rising or high O antibody titers generally indicate acute infection. The H antibody appears slightly later and persists longer. The elevations of H antibody help to identify the type of enteric fever. Numerous studies have shown that the sensitivity, specificity, and predictive values of Widal test vary rendering the test's value to the clinician questionable. This wide variation is caused by differences in patients’ stages of infection and antigens variations, The Widal reaction is indicative of typhoid fever in only 4060% of patients. These are other serological method is available such as ELISA method (enzymelinked immunosorbent assay) for immunoglobulin M (IgM) and immunoglobulin G antibodies to S typhi polysaccharide, the monoclonal antibodies against S typhi flagellin and DNA probes and these are confirmatory test. The other lab. tests (Blood culture) Clinical diagnosis is supported by tests that identify Salmonella antibodies and is then confirmed by isolation of the organism and this is done by blood culture. The definitive diagnosis of typhoid fever requires isolation of the organism from blood or bone marrow. With culture-positive rates of approximately 90%, the most sensitive method of isolating S typhi is obtaining a bone marrow aspirate (BMA) culture. BMA and blood are cultured in a selective medium, such as 10% aqueous oxgall, or a nutritious medium, such as tryptic soy broth, and are incubated at 37°C for at least 7 days before reporting negative. Subcultures are made daily to one selective medium, such as MacConkey agar, and one inhibitory medium, such as Salmonella-Shigella agar 40 Some illustrations of pictures of laboratory concerns 41 42 43 Blood agar plate Different hemolytic reactions Staphylococcus aureus showing beta haemolysis in Blood agar and colonial characteristic in Nutrient agar 44 Lactose fermenters will appear pink, non-lactose fermenters will appear colorless 45 Triple Sugar Iron Agar (TSI) TSI agar contains peptone, glucose, sucrose, lactose, and thiosulfate 46 MOTILITY TEST • OrganismTest Result • 1. Ps aeruginosa • 2. S aureus • 3. B subtilis Motile Non-motile Motile Egg of Ascaris lumbricoides, Ankylostoma duodenale, Enterobius vermacularis, Trichuris trichuria 47 Egg of Taena and proglottid of T sagina & T solium egg of Hymenolepis nana Trophozoite and cyst Giardia lamblia & Entamoeba hystolytica 48 49 Microfilaria bancrofti and brugia 50 Ziehl Neelson’s staining (AFB Staining) Continued AFB Staining 51 Mucor / Penicillium / Aspergillus Microsporum gypseum & Trichophyton mentagrophytes 52 53 54 55 56