File
advertisement
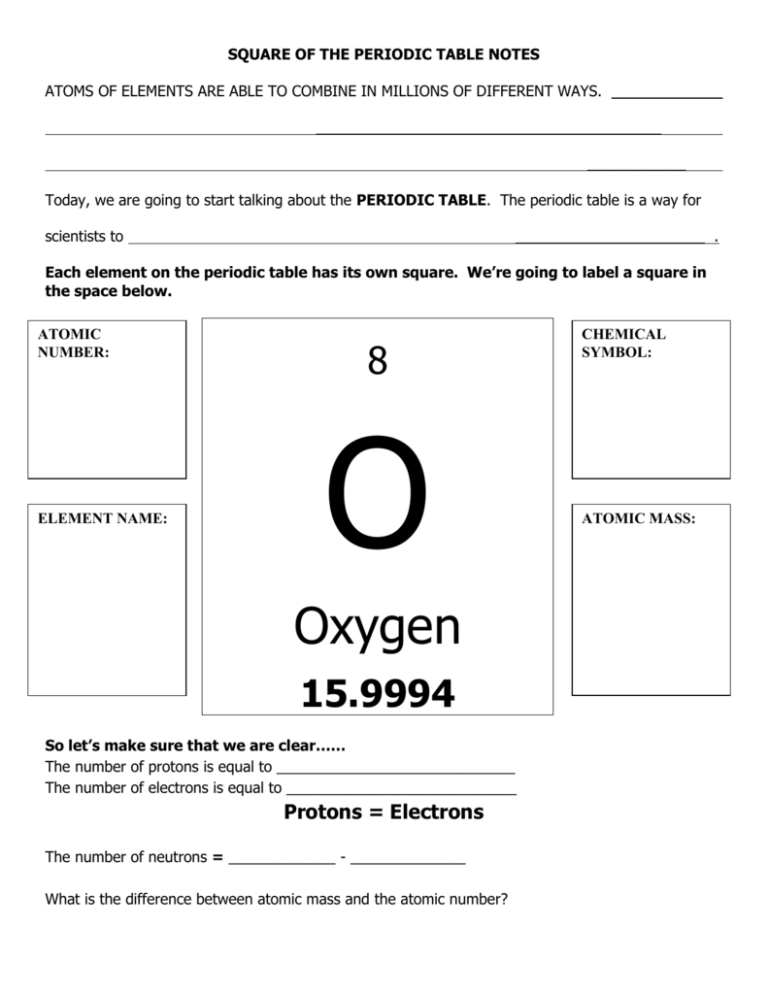
SQUARE OF THE PERIODIC TABLE NOTES ATOMS OF ELEMENTS ARE ABLE TO COMBINE IN MILLIONS OF DIFFERENT WAYS. __________________________________________ ____________ Today, we are going to start talking about the PERIODIC TABLE. The periodic table is a way for scientists to _______________________ . Each element on the periodic table has its own square. We’re going to label a square in the space below. ATOMIC NUMBER: ELEMENT NAME: 8 O Oxygen 15.9994 So let’s make sure that we are clear…… The number of protons is equal to _____________________________ The number of electrons is equal to ____________________________ Protons = Electrons The number of neutrons = _____________ - ______________ What is the difference between atomic mass and the atomic number? CHEMICAL SYMBOL: ATOMIC MASS: We are going to spend some time looking at the periodic table and answering questions about it—we need to make sure that we understand EXACTLY what we’re looking at when we see one square of the periodic table. Oxygen What is the chemical symbol for oxygen? What is the atomic number of the element oxygen? What is the atomic mass of the element oxygen? How many protons does oxygen have? How many neutrons does oxygen have? Lithium What is the chemical symbol for lithium? What is the atomic number of the element lithium? What is the atomic mass of the element lithium? How many protons does lithium have? How many neutrons does lithium have? Calcium What is the chemical symbol for calcium? What is the atomic number of the element calcium? What is the atomic mass of the element calcium? How many protons does calcium have? How many neutrons does calcium have? Strontium What is the chemical symbol for strontium? What is the atomic number of the element strontium? What is the atomic mass of the element strontium? How many protons does strontium have? How many neutrons does strontium have?