Antacid Effectiveness
advertisement
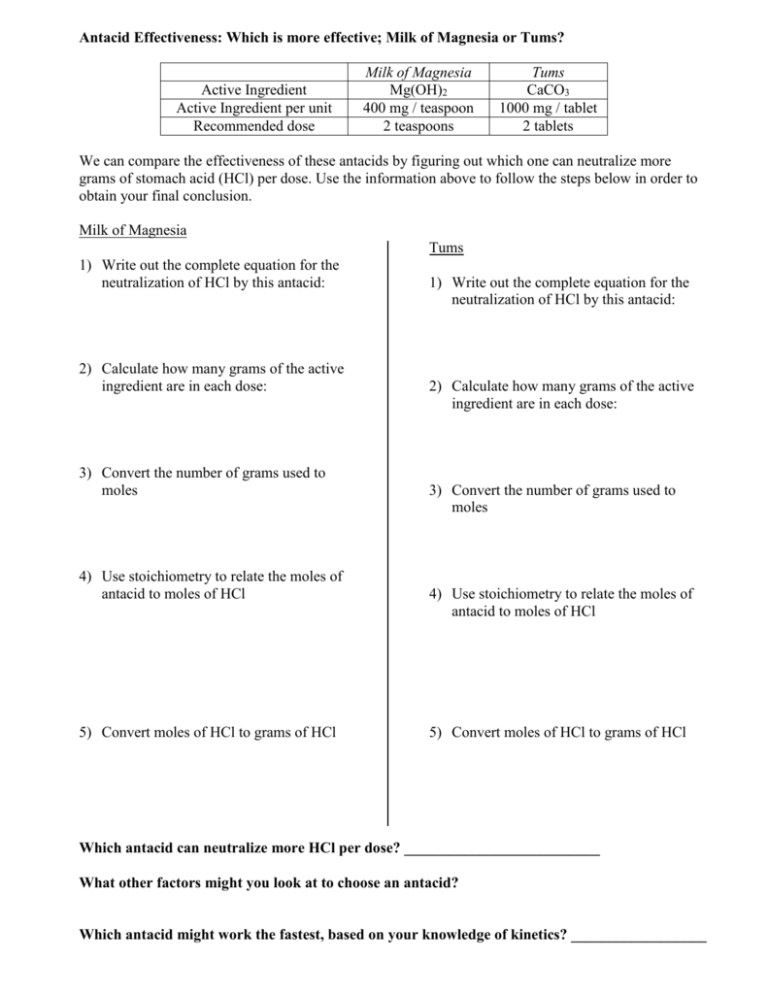
Antacid Effectiveness: Which is more effective; Milk of Magnesia or Tums? Active Ingredient Active Ingredient per unit Recommended dose Milk of Magnesia Mg(OH)2 400 mg / teaspoon 2 teaspoons Tums CaCO3 1000 mg / tablet 2 tablets We can compare the effectiveness of these antacids by figuring out which one can neutralize more grams of stomach acid (HCl) per dose. Use the information above to follow the steps below in order to obtain your final conclusion. Milk of Magnesia Tums 1) Write out the complete equation for the neutralization of HCl by this antacid: 2) Calculate how many grams of the active ingredient are in each dose: 3) Convert the number of grams used to moles 4) Use stoichiometry to relate the moles of antacid to moles of HCl 5) Convert moles of HCl to grams of HCl 1) Write out the complete equation for the neutralization of HCl by this antacid: 2) Calculate how many grams of the active ingredient are in each dose: 3) Convert the number of grams used to moles 4) Use stoichiometry to relate the moles of antacid to moles of HCl 5) Convert moles of HCl to grams of HCl Which antacid can neutralize more HCl per dose? __________________________ What other factors might you look at to choose an antacid? Which antacid might work the fastest, based on your knowledge of kinetics? __________________










