Chemistry Molarity Lab Sheet 4-23-12
advertisement

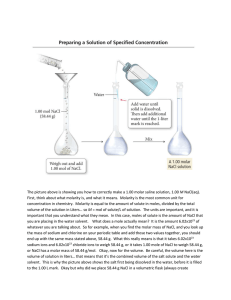
Chemistry Molarity Lab Sheet 4-23-12 *Materials - solid NaCl - water - 50mL volumetric flask - electronic balance Procedure - Measure the mass of a clean, dry volumetric flask. - Add 30-35g of NaCl to the flask. - Measure the mass of the flask again - Half fill the flask with tap water and shake it gently until all the NaCl has been dissolved. - Fill the flask with water to the 50mL mark and measure the mass again. - Create a data table to record these results. (no I won’t help you with the table! I am quite certain you can construct one yourself…) Analyze: Enter the following equations into your SIS then use the data from your table above to answer the following questions…. 1. % by mass tells how many grams of solute are present in 100g of solution. Equation: % by mass = mass of solute mass of solute + solvent (mass of solution) **Calculate the % by mass of NaCl in the solution 2. Mole fraction tells how many moles of solute are present for every 1mol of total solution. Equation: mole fraction = mol NaCl mol NaCl + mol H2O **Calculate the mole fraction of your solution 3. Molality tells how many moles of solute are present in 1kg of solvent Equation: molality (m) = mol NaCl kg of H2O **calculate the molality of your solution 4. Molarity (M) tells how many moles of solute are dissolved in 1L of solution. Equation: molarity (M) = mol NaCl L of solution **Calculate the molality of your NaCl solution 5. Density tells how many grams of solution are present in 1mL of solution Equation: density = g of solution mL of solution **Calculate the density of your solution