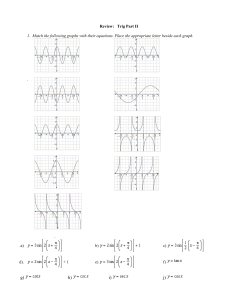
Chemistry Calculations Test Bank: Formulas & Equations
advertisement
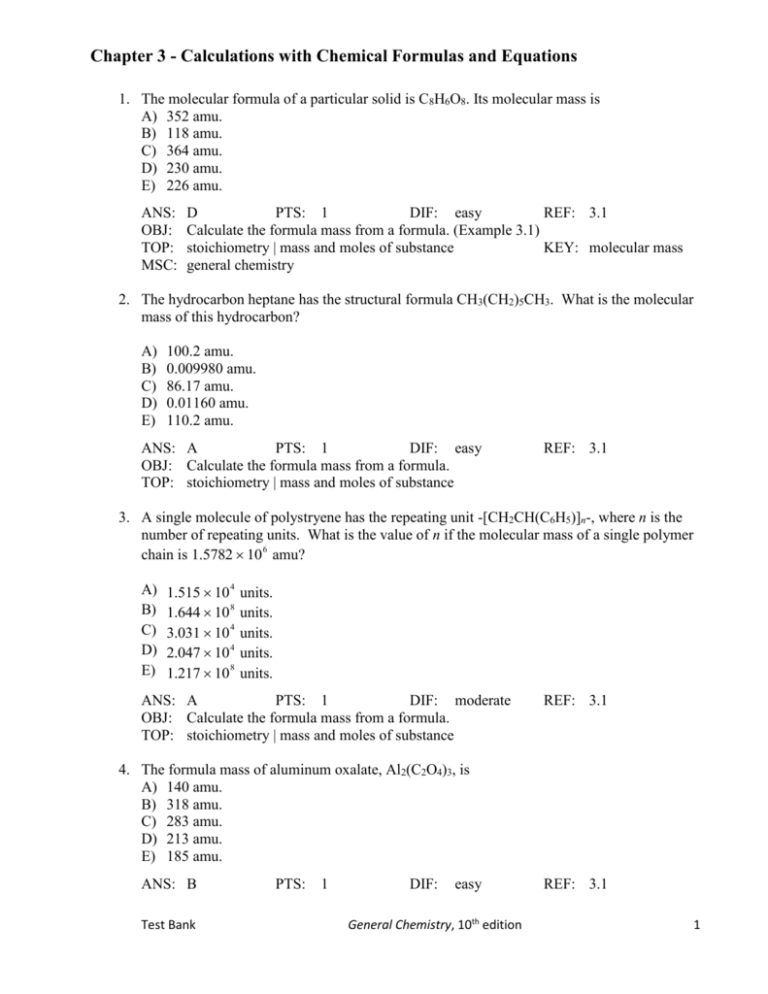
Chapter 3 - Calculations with Chemical Formulas and Equations 1. The molecular formula of a particular solid is C8H6O8. Its molecular mass is A) 352 amu. B) 118 amu. C) 364 amu. D) 230 amu. E) 226 amu. ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy REF: 3.1 Calculate the formula mass from a formula. (Example 3.1) stoichiometry | mass and moles of substance KEY: molecular mass general chemistry 2. The hydrocarbon heptane has the structural formula CH3(CH2)5CH3. What is the molecular mass of this hydrocarbon? A) B) C) D) E) 100.2 amu. 0.009980 amu. 86.17 amu. 0.01160 amu. 110.2 amu. ANS: A PTS: 1 DIF: easy OBJ: Calculate the formula mass from a formula. TOP: stoichiometry | mass and moles of substance REF: 3.1 3. A single molecule of polystryene has the repeating unit -[CH2CH(C6H5)]n-, where n is the number of repeating units. What is the value of n if the molecular mass of a single polymer chain is 1.5782 10 6 amu? A) B) C) D) E) 1.515 10 4 1.644 10 8 3.031 10 4 2.047 10 4 1.217 10 8 units. units. units. units. units. ANS: A PTS: 1 DIF: moderate OBJ: Calculate the formula mass from a formula. TOP: stoichiometry | mass and moles of substance REF: 3.1 4. The formula mass of aluminum oxalate, Al2(C2O4)3, is A) 140 amu. B) 318 amu. C) 283 amu. D) 213 amu. E) 185 amu. ANS: B Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.1 1 OBJ: Calculate the formula mass from a formula. (Example 3.1) TOP: stoichiometry | mass and moles of substance KEY: formula mass MSC: general chemistry 5. The formula mass of zinc acetate trihydrate, Zn(CH3COO)2 • 3H2O, is A) 321 amu. B) 184 amu. C) 268 amu. D) 238 amu. E) 114 amu. ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy REF: 3.1 Calculate the formula mass from a formula. (Example 3.1) stoichiometry | mass and moles of substance KEY: formula mass general chemistry 6. The fully hydrated form of sodium sulfate is the decahydrate, Na2SO4 • 10H2O. When heated the hydrated salt loses water. How many water molecules are found per formula unit in a partially dehydrated sample of sodium sulfate with a formula mass of 160.1 amu (i.e. find n for Na2SO4 • nH2O)? A) B) C) D) E) 1 waters. 5 waters. 3 waters. 4 waters. 7 waters. ANS: A PTS: 1 DIF: moderate OBJ: Calculate the formula mass from a formula. TOP: stoichiometry | mass and moles of substance REF: 3.1 7. What is the molecular mass of cycloheptane, C7H14? A) 13.02 amu B) 1191.19 amu C) 85.08 amu D) 98.19 amu E) 26.12 amu ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy REF: 3.1 Calculate the formula mass from a formula. (Example 3.1) stoichiometry | mass and moles of substance KEY: molecular mass general chemistry 8. What is the formula mass of strontium phosphate, Sr3(PO4)2? A) 357.83 amu B) 421.83 amu C) 182.59 amu D) 715.66 amu E) 452.80 amu ANS: E Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.1 2 OBJ: Calculate the formula mass from a formula. (Example 3.1) TOP: stoichiometry | mass and moles of substance KEY: formula mass MSC: general chemistry 9. What is the molecular mass of the hydrocarbon styrene (shown in the figure)? A) B) C) D) E) 104.1 amu. 91.1 amu. 103.1 amu. 13.0 amu. 78.1 amu. ANS: OBJ: TOP: MSC: A PTS: 1 DIF: easy REF: 3.1 Calculate the formula mass from molecular models. (Example 3.2) stoichiometry | mass and moles of substance KEY: molecular mass general chemistry 10. What is the molar mass of ammonium sulfite, (NH4)2SO3? A) 98 g/mol B) 116 g/mol C) 55 g/mol D) 180 g/mol E) 84 g/mol ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.2 Understand how the molar mass is related to the formula weight of a substance. stoichiometry | mass and moles of substance KEY: formula mass general chemistry 11. What is the molar mass of zinc sulfate heptahydrate, ZnSO4 • 7H2O? A) 180. g/mol B) 288 g/mol C) 384 g/mol D) 162 g/mol E) 582 g/mol ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.2 Understand how the molar mass is related to the formula weight of a substance. stoichiometry | mass and moles of substance KEY: formula mass general chemistry Test Bank General Chemistry, 10th edition 3 12. Plastic wrap can be made from poly(vinylidene chloride). A single poly(vinylidene chloride) strand has the general formula -(CH2CHCl)n-, where n ranges from 10,000 to 100,000. What is the molar mass of a single poly(vinylidene chloride) molecule containing 6.928 10 4 repeating units? A) B) C) D) E) 4.330 10 6 g/mol 2.706 10 6 g/mol 2.310 10 7 g/mol 6.308 10 6 g/mol 1.585 10 7 g/mol ANS: A PTS: 1 DIF: easy REF: 3.2 OBJ: Understand how the molar mass is related to the formula weight of a substance. TOP: stoichiometry | mass and moles of substance 13. The dicarboxylic acid potassium hydrogen pthalate (shown in the figure) is used to standardize solutions of strong base. What is the molar mass of this compound? A) B) C) D) E) 204.2 g/mol 192.2 g/mol 248.9 g/mol 71.08 g/mol 172.2 g/mol ANS: A PTS: 1 DIF: easy REF: 3.2 OBJ: Understand how the molar mass is related to the formula weight of a substance. TOP: stoichiometry | mass and moles of substance 14. What is the molar mass of the solid C8H12N2O4? A) 172 g/mol B) 136 g/mol C) 200 g/mol D) 106 g/mol E) 188 g/mol ANS: OBJ: TOP: MSC: C PTS: 1 DIF: easy REF: 3.2 Understand how the molar mass is related to the formula weight of a substance. stoichiometry | mass and moles of substance KEY: molecular mass general chemistry 15. A 1.067 g sample of an element contains 5.062 1021 atoms. What is the element symbol? A) I Test Bank General Chemistry, 10th edition 4 B) C) D) E) Ag La Pd Rh ANS: A PTS: 1 DIF: moderate REF: 3.2 OBJ: Understand how the molar mass is related to the formula weight of a substance. TOP: stoichiometry | determining chemical formulas 16. Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN)n, where n is typically greater than 10,000. Given that a sample of monodisperse polyacrilonitrile weighs 197.4 g and contains 1.046 10 20 molecules of -(CH2CHCN)n-, calculate n. A) B) C) D) E) 2.141 10 4 6.026 10 7 6.018 10 39 6.022 10 23 1.136 10 6 ANS: A PTS: 1 DIF: difficult REF: 3.2 OBJ: Understand how the molar mass is related to the formula weight of a substance. TOP: stoichiometry | mass and moles of substance 17. Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN)n, where n is typically greater than 10,000. Given that a sample of monodisperse polyacrylonitrile weighs 252.6 g and contains 4.443 10 19 molecules of -(CH2CHCN)n-, what is the molar mass of the polymer? A) B) C) D) E) 6.452 10 4 g/mol 1.816 10 8 g/mol 1.997 10 39 g/mol 6.022 10 23 g/mol 3.423 10 6 g/mol ANS: E PTS: 1 DIF: difficult REF: 3.2 OBJ: Understand how the molar mass is related to the formula weight of a substance. TOP: stoichiometry | mass and moles of substance 18. An atom of an element weighs 3.00 10–23 g. What is the atomic mass of this element in atomic mass units? A) 17.4 amu B) 16.5 amu C) 15.9 amu D) 18.1 amu E) 15.3 amu ANS: D PTS: 1 DIF: easy REF: 3.2 OBJ: Calculate the mass of atoms and molecules. (Example 3.3) Test Bank General Chemistry, 10th edition 5 TOP: stoichiometry | mass and moles of substance KEY: mole | mole calculations MSC: general chemistry 19. What is the mass in grams of one propene, C3H6, molecule? A) 6.99 10–23 g B) 2.53 1025 g C) 44.0 g D) 42.0 g E) 1.99 10–23 g ANS: OBJ: TOP: KEY: A PTS: 1 DIF: easy REF: 3.2 Calculate the mass of atoms and molecules. (Example 3.3) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 20. What is the mass of oxygen in grams found in one molecule of the compound C7H8O4? A) B) C) D) E) 1.06 10 22 g 3.86 10 21 g 1.34 10 23 g 1.40 10 22 g 156 g ANS: A PTS: 1 DIF: moderate REF: 3.2 OBJ: Calculate the mass of atoms and molecules. (Example 3.3) TOP: stoichiometry | mass and moles of substance 21. Which of the following compounds contains the largest number of atoms? A) 4.0 mol of K2S B) 3.0 mol of NH3 C) 2.0 mol of H2SO4 D) 5.0 mol of HCl E) 1.0 mol of CH3COCl ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.2 Perform calculations using the mole. stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 22. How many atoms of carbon are there in 0.51 mol of procaine, C13H20N2O2, a “pain killer” used by dentists? A) 6.6 1023 B) 4.3 1024 C) 4.0 1024 D) 6.1 1023 E) 4.6 1024 ANS: C PTS: 1 DIF: easy OBJ: Perform calculations using the mole. TOP: stoichiometry | mass and moles of substance Test Bank General Chemistry, 10th edition REF: 3.2 6 KEY: mole | mole calculations MSC: general chemistry 23. A sample of ammonium phosphite, (NH4)3PO3, contains 0.909 mol of hydrogen atoms. The number of moles of oxygen atoms in the sample is A) B) C) D) E) 0.227 mol. 0.0909 mol. 0.300 mol. 0.227 mol. 3.00 mol. ANS: A PTS: 1 DIF: easy OBJ: Perform calculations using the mole. TOP: stoichiometry | mass and moles of substance REF: 3.2 24. A sample of gallium(III) sulfite, Ga2(SO3)3, contains 1.95 mol of sulfite ions. The number of moles of gallium(III) ions in the sample is A) B) C) D) E) 1.30 mol. 2.92 mol. 1.95 mol. 5.84 mol. 3.90 mol. ANS: A PTS: 1 DIF: easy OBJ: Perform calculations using the mole. TOP: stoichiometry | mass and moles of substance REF: 3.2 25. Sorbose, C6H12O6, is used in making vitamin C. A sorbose sample containing 96.0 g of carbon atoms also contains ____ g of hydrogen atoms. A) 32.2 B) 16.1 C) 1.15 103 D) 96.0 E) 7.99 ANS: OBJ: TOP: KEY: B PTS: 1 DIF: moderate REF: 3.2 Perform calculations using the mole. stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 26. Consider the following three samples. A. A sample containing 180 g glucose (C6H12O6) B. A sample containing 90 g glucose and 90 g fructose (C6H12O6) C. A sample containing 180 g fructose Which statement is correct? A) All three samples have the same number of hydrogen atoms. B) Both samples A and C have the same number of hydrogen atoms, but more than in sample B. C) Sample B has more hydrogen atoms than sample A or sample C. Test Bank General Chemistry, 10th edition 7 D) Sample C has more hydrogen atoms than sample A or sample B E) Sample A has more hydrogen atoms than sample B or sample C. ANS: OBJ: TOP: KEY: A PTS: 1 DIF: difficult REF: 3.2 Perform calculations using the mole. stoichiometry | determining chemical formulas mole | mole calculations MSC: general chemistry 27. Styrene's empirical formula is CH. What mass of styrene contains 7.89 1021 atoms of hydrogen? The molar mass of styrene is 104 g/mol. A) 0.105 g B) 0.170 g C) 1.36 g D) 0.157 g E) 0.0131 g ANS: OBJ: TOP: KEY: B PTS: 1 DIF: moderate REF: 3.2 Convert from moles of substance to grams of substance. (Example 3.4) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 28. What is the mass of oxygen atoms in 0.305 mol Fe(CO)5? A) 17.0 g B) 59.7 g C) 24.4 g D) 4.88 g E) 18.3 g ANS: OBJ: TOP: KEY: C PTS: 1 DIF: moderate REF: 3.2 Convert from moles of substance to grams of substance. (Example 3.4) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 29. What is the mass in grams of 0.699 mol of glucose, C6H12O6? A) 0.00388 g B) 67.1 g C) 126 g D) 21.0 g E) 258 g ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.2 Convert from moles of substance to grams of substance. (Example 3.4) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 30. Which one of the following samples has the greatest mass? A) 0.39 mol of camphor, C10H16O B) 4.1 mol of ammonia, NH3 C) 9.2 mol of krypton, Kr D) 4.6 mol of iodine vapor, I2 E) 1.8 mol of formaldehyde, CH2O Test Bank General Chemistry, 10th edition 8 ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.2 Convert from moles of substance to grams of substance. (Example 3.4) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 31. Which of the following contains the greatest mass of oxygen atoms? A) 0.6 mol CoSO4 • 7H2O B) 2.3 mol KHSO4 C) 1.2 mol K2Cr2O7 D) 2.3 mol H2O2 E) 2.3 mol Na2S2O3 ANS: OBJ: TOP: KEY: B PTS: 1 DIF: easy REF: 3.2 Convert from moles of substance to grams of substance. (Example 3.4) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 32. Calculate the number of moles of bromine present in 14.5 mL of Br2(l), whose density is 3.12 g/mL. A) 3.53 mol B) 0.181 mol C) 0.566 mol D) 0.091 mol E) 0.283 mol ANS: OBJ: TOP: KEY: E PTS: 1 DIF: moderate REF: 3.2 Convert from grams of substance to moles of substance. (Example 3.5) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 33. How many moles of hexachlorobenzene, C6Cl6, are in 4.45 g of C6Cl6? A) 0.0208 mol B) 1.27 103 mol C) 0.0618 mol D) 0.0156 mol E) 0.0322 mol ANS: OBJ: TOP: KEY: D PTS: 1 DIF: easy REF: 3.2 Convert from grams of substance to moles of substance. (Example 3.5) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 34. How many moles of iron atoms are contained in 4.39 g of iron? A) 245 mol B) 0.0579 mol C) 0.0786 mol D) 0.122 mol E) 0.169 mol ANS: C Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.2 9 OBJ: Convert from grams of substance to moles of substance. (Example 3.5) TOP: stoichiometry | mass and moles of substance KEY: mole | mole calculations MSC: general chemistry 35. How many moles of pentane, C5H12, are contained in a 11-g sample? A) 0.18 mol B) 0.15 mol C) 0.26 mol D) 1.4 mol E) 1.1 mol ANS: OBJ: TOP: KEY: B PTS: 1 DIF: easy REF: 3.2 Convert from grams of substance to moles of substance. (Example 3.5) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 36. Sodium cyclamate, C6H11NHSO3Na, was used at one time as an artificial sweetener. C6H11NHSO3Na has a molecular mass of 201.2 g/mol. How many moles of sodium cyclamate are contained in a 67.6-g sample? A) 0.211 mol B) 0.193 mol C) 0.336 mol D) 0.307 mol E) 13,600 mol ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.2 Convert from grams of substance to moles of substance. (Example 3.5) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 37. How many moles of silver are contained in 7.00 kg of silver? A) 64.9 mol B) 64.9 101 mol C) 64.9 10–3 mol D) 64.9 103 mol E) 64.9 10–1 mol ANS: OBJ: TOP: KEY: A PTS: 1 DIF: easy REF: 3.2 Convert from grams of substance to moles of substance. (Example 3.5) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 38. A 0.0103-mol sample of urea, NH2CONH2, contains A) 6.02 1023 molecules. B) 2.48 1022 molecules. C) 4.96 1022 atoms. D) 2.48 1023 atoms. E) 1.03 1024 atoms. ANS: C Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.2 10 OBJ: Calculate the number of molecules in a given mass of substance. (Example 3.6) TOP: stoichiometry | mass and moles of substance KEY: mole | mole calculations MSC: general chemistry 39. How many molecules are there in 90.0 g of butylene glycol, HO(CH2)4OH? A) 1 B) (6.02 1023)/90.0 C) 90.0 D) 90.0 (6.02 1023) E) 6.02 1023 ANS: OBJ: TOP: KEY: E PTS: 1 DIF: easy REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 40. How many molecules are there in 192 g of citric acid, C6H8O7? A) (6.02 1023) / 192 B) 192 C) 6.02 1023 D) 96.0 E) 192 (6.02 1023) ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 41. In 0.358 mol of trimellitic acid, C6H3(COOH)3, there are A) 2.16 1022 molecules. B) 8.62 1024 molecules. C) 6.47 1023 oxygen atoms. D) 1.94 1024 carbon atoms. E) 3.59 1023 hydrogen atoms. ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 42. How many molecules are there in 2.80 kg of hydrazine, N2H4? A) 8.75 1023 B) 5.27 1025 C) 1.88 1022 D) 2.80 1026 E) 4.65 1021 ANS: B PTS: 1 DIF: moderate REF: 3.2 OBJ: Calculate the number of molecules in a given mass of substance. (Example 3.6) TOP: stoichiometry | mass and moles of substance Test Bank General Chemistry, 10th edition 11 KEY: mole | mole calculations MSC: general chemistry 43. How many molecules are there in 2.43 mg of mannose, C6H12O6, which is a sweet-tasting sugar that has a bitter aftertaste? A) 4.46 1021 B) 2.48 1021 C) 7.31 1018 D) 4.04 1024 E) 8.13 1018 ANS: OBJ: TOP: KEY: E PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 44. How many atoms are present in 463 g of KPF6 (MM = 184.1 g/mol)? A) 2.23 1025 B) 1.51 1021 C) 2.51 1021 D) 1.13 1026 E) 1.21 1025 ANS: OBJ: TOP: KEY: E PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 45. Styrene's empirical formula is CH. When it is heated to 200°C, it is converted into a polymer, polystyrene, which has excellent insulating properties. What mass of styrene contains 9.75 1021 molecules of styrene? The molar mass of styrene is 104 g/mol. A) 0.0162 g B) 1.68 g C) 3.24 g D) 0.210 g E) 0.130 g ANS: OBJ: TOP: KEY: B PTS: 1 DIF: difficult REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 46. In 0.500 mol of dimethylhydrazine, (CH3)2N2H2, there are A) 3.01 1024 molecules. B) 1.51 1023 atoms. C) 3.01 1022 molecules. D) 3.61 1024 atoms. E) 1.81 1024 atoms. ANS: D PTS: 1 DIF: moderate REF: 3.2 OBJ: Calculate the number of molecules in a given mass of substance. (Example 3.6) Test Bank General Chemistry, 10th edition 12 TOP: stoichiometry | mass and moles of substance KEY: mole | mole calculations MSC: general chemistry 47. Which is a reasonable mass corresponding to 1020 molecules of a substance? A) 100 g B) 100 µg C) 100 ng D) 100 mg E) 100 kg ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 48. How many aluminum atoms are there in 52 g of Al2S3? A) 4.2 1023 B) 1.6 1021 C) 2.1 1023 D) 1.1 1021 E) 6.3 1023 ANS: OBJ: TOP: KEY: A PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 49. Which one of the following contains 2.41 1024 atoms? A) 52.0 g C2H2 B) 96.0 g O2 C) 32.0 g CH4 D) 168 g N2 E) 16.0 g He ANS: OBJ: TOP: KEY: E PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 50. A sample of 496 g of white phosphorus, P4, contains the same number of atoms as A) 192 g of ozone (O3). B) 56.0 g of nitrogen (N2). C) 92.0 g of sodium. D) 120 g of formaldehyde (CH2O). E) 128 g of oxygen (O2). ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry Test Bank General Chemistry, 10th edition 13 51. The total number of oxygen atoms in 1.76 g of CaCO3 (MM = 100.0 g/mol) is A) 2.05 1023. B) 4.24 1022. C) 3.18 1022. D) 1.75 1023. E) 5.30 1022. ANS: OBJ: TOP: KEY: C PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 52. A sample of 336 g of ozone, O3, contains the same number of atoms as A) 336 g of oxygen (O2). B) 28.2 g of hydrogen (H2). C) 266 g of fluorine (F2). D) 189 g of aluminum (Al). E) 411 g of nickel (Ni). ANS: OBJ: TOP: KEY: A PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 53. Which of the following samples contains the smallest number of molecules? A) 8.00 g of TNT, C7H5N3O6 B) 8.00 g of benzene, C6H6 C) 8.00 g of glucose, C6H12O6 D) 8.00 g of naphthalene, C10H8 E) 8.00 g of formaldehyde, CH2O ANS: OBJ: TOP: KEY: A PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 54. Which of the following samples contains the largest number of atoms? A) 1 g N2 B) 1 g Li C) 1 g Cl2 D) 1 g P4 E) 1 g Mg ANS: OBJ: TOP: KEY: B PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 55. Which of the following samples contains the largest number of molecules? Test Bank General Chemistry, 10th edition 14 A) B) C) D) E) 10. g Pb 10. g Cl2 10. g Kr 10. g O2 10. g S8 ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.2 Calculate the number of molecules in a given mass of substance. (Example 3.6) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 56. In 1928, 1.0 g of rhenium, Re, was isolated from 660 kg of the ore molybenite. The percent by mass of this element in the molybenite was A) 0.66 %. B) 0.15 %. C) 3.5 10–4 %. D) 6.6 10–3 %. E) 1.5 10–4 %. ANS: OBJ: TOP: MSC: E PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 57. An ore sample is found to contain 24.1 g of mercury and 50.7 g waste rock (gangue). What is the percent by mass of mercury in the ore? A) B) C) D) E) 32.2 % 47.4 % 0.322 % 0.474 % 4.74 % ANS: A PTS: 1 DIF: easy REF: 3.3 OBJ: Calculate the percentage composition of the elements in a compound. (Example 3.7) TOP: stoichiometry | determining chemical formulas 58. An ore sample with a mass of 68.0 g is found to contain 15.5% by mass nickel. What mass of nickel is contained in the ore? A) B) C) D) E) 10.5 g 81.7 g 1.55 g 84.5 g 546 g ANS: A PTS: 1 DIF: easy REF: 3.3 OBJ: Calculate the percentage composition of the elements in a compound. (Example 3.7) TOP: stoichiometry | determining chemical formulas 59. What is the percent by mass oxygen in (NH4)2SO3? Test Bank General Chemistry, 10th edition 15 A) B) C) D) E) 41.3 % 20.7 % 54.0 % 42.0 % 1.00 % ANS: A PTS: 1 DIF: easy REF: 3.3 OBJ: Calculate the percentage composition of the elements in a compound. (Example 3.7) TOP: stoichiometry | determining chemical formulas 60. What is the percentage by mass of hydrogen in the insecticide Lindane, C6H6Cl6? A) B) C) D) E) 20.0 % 1.20 % 47.2 % 8.80 % 2.08 % ANS: E PTS: 1 DIF: easy REF: 3.3 OBJ: Calculate the percentage composition of the elements in a compound. (Example 3.7) TOP: stoichiometry | determining chemical formulas 61. The mineral leadhillite, which is essentially Pb4(SO4)(CO3)2(OH)2 (FW = 1079 g/mol), contains ____% hydrogen by mass. A) 76.81 B) 0.1868 C) 2.226 D) 17.79 E) 2.972 ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 62. Which of the following compounds has the highest percentage of hydrogen atoms by mass? A) CH3COOH B) C2H5OH C) CH3OH D) H2CO3 E) H2C2O4 ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 63. Which of the following compounds has the highest percentage of nitrogen by mass? A) (NH4)2SO3 B) NaNO3 Test Bank General Chemistry, 10th edition 16 C) N2Cl4 D) NH4NO2 E) HNO3 ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 64. Which of the following compounds has the same percentage of carbon and hydrogen by mass as cyclohexane, C6H12? A) C6H14, hexane B) C5H10, pentene C) C6H10, cyclohexene D) C6H6, benzene E) C6H12O6, glucose ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 65. What is the mass percentage of carbon in the compound C6H6O2? A) 5.5 % B) 70.9 % C) 65.5 % D) 29.1 % E) 14.3 % ANS: OBJ: TOP: MSC: C PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 66. What is the percentage by mass of hydrogen in ammonium phosphate, (NH4)3PO4? A) B) C) D) E) 8.11 % 4.06 % 52.0 % 40.0 % 3.00 % ANS: A PTS: 1 DIF: easy REF: 3.3 OBJ: Calculate the percentage composition of the elements in a compound. (Example 3.7) TOP: stoichiometry | determining chemical formulas 67. A crystal of the mineral troegerite, (UO2)3(AsO4)2 • 12H2O (FM = 1304 amu), contains ____% arsenic by mass. A) 15.4 B) 39.8 C) 61.0 Test Bank General Chemistry, 10th edition 17 D) 26.4 E) 11.5 ANS: OBJ: TOP: MSC: E PTS: 1 DIF: easy REF: 3.3 Calculate the percentage composition of the elements in a compound. (Example 3.7) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 68. How many grams of hydrogen atoms are present in 18.4 g of water? A) 37.1 g B) 1.02 g C) 2.06 g D) 1.96 g E) 12.3 g ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.3 Calculate the mass of an element in a given mass of compound. (Example 3.8) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 69. How many grams of potassium are present in 21.6 g of K2Cr2O7? A) 5.74 g B) 1.105 g C) 2.87 g D) 78.2 g E) 10.8 g ANS: OBJ: TOP: MSC: A PTS: 1 DIF: moderate REF: 3.3 Calculate the mass of an element in a given mass of compound. (Example 3.8) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 70. NaHCO3 is the active ingredient in baking soda. How many grams of oxygen are present in 0.67 g of NaHCO3? A) 0.024 g B) 0.128 g C) 7.98 103 g D) 0.043 g E) 0.38 g ANS: OBJ: TOP: MSC: E PTS: 1 DIF: easy REF: 3.3 Calculate the mass of an element in a given mass of compound. (Example 3.8) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 71. Which of the following contains the greatest mass of bromine atoms? A) 11.0 g of KBr B) 23.0 g of Br2 C) 0.076 mol of KBr D) 0.092 mol of Br2 E) 32.0 g of NaBrO3 Test Bank General Chemistry, 10th edition 18 ANS: OBJ: TOP: MSC: B PTS: 1 DIF: moderate REF: 3.3 Calculate the mass of an element in a given mass of compound. (Example 3.8) stoichiometry | determining chemical formulas KEY: mass percentage general chemistry 72. The amount of calcium in a 15.0-g sample was determined by converting the calcium to calcium oxalate, CaC2O4. The CaC2O4 weighed 12.6 g. What is the percent of calcium in the original sample? A) 10.8 % B) 26.3 % C) 14.8 % D) 33.7 % E) 84.0 % ANS: OBJ: TOP: KEY: B PTS: 1 DIF: difficult REF: 3.3 Calculate the mass of an element in a given mass of compound. (Example 3.8) stoichiometry | mass and moles of substance mole | mole calculations MSC: general chemistry 73. A compound containing only carbon, hydrogen, and oxygen is subjected to elemental analysis. Upon complete combustion, a 0.1804-g sample of the compound produced 0.3051 g of CO2 and 0.1249 g of H2O. What is the empirical formula of the compound? A) C3H6O3 B) C3H3O C) C4H8O3 D) C2H2O E) CH2O3 ANS: OBJ: TOP: MSC: C PTS: 1 DIF: moderate REF: 3.4 Calculate the percentage of C, H, and O from combustion data. (Example 3.9) stoichiometry | determining chemical formulas KEY: elemental analysis general chemistry 74. A 3.075 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess of dioxygen, producing 6.990 g CO2 and 2.862 g H2O. What mass of oxygen is contained in the original sample? A) B) C) D) E) 0.8472 g 1.167 g 3.915 g 4.129 g 0.2134 g ANS: A PTS: 1 DIF: moderate REF: 3.4 OBJ: Calculate the percentage of C, H, and O from combustion data. (Example 3.9) TOP: stoichiometry | determining chemical formulas 75. A 4.043 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess of dioxygen, producing 9.191 g CO2 and 3.762 g H2O. What percent by mass of oxygen is contained in the original sample? Test Bank General Chemistry, 10th edition 19 A) B) C) D) E) 27.54 % 37.96 % 12.73 % 13.43 % 6.939 % ANS: A PTS: 1 DIF: moderate REF: 3.4 OBJ: Calculate the percentage of C, H, and O from combustion data. TOP: stoichiometry | determining chemical formulas 76. A 2.841 g sample of a hydrocarbon is burned in an excess of dioxygen, producing 7.794 g CO2 and water. What mass of hydrogen is contained in the original sample? A) B) C) D) E) 0.7140 g 4.953 g 10.64 g 2.826 g 1.421 g ANS: A PTS: 1 DIF: moderate REF: 3.4 OBJ: Calculate the percentage of C, H, and O from combustion data. (Example 3.9) TOP: stoichiometry | determining chemical formulas 77. A 2.445 g sample of a hydrocarbon is burned in an excess of dioxygen, producing 6.708 g CO2 and 5.492 g H2O. What is the empirical formula of the hydrocarbon? A) B) C) D) E) CH4 CH2 C2H3 CH3 CH ANS: A PTS: 1 DIF: moderate OBJ: Determine the empirical formula of a binary compound. TOP: stoichiometry | determining chemical formulas REF: 3.5 78. A 0.4647-g sample of a compound known to contain only carbon, hydrogen, and oxygen was burned in dioxygen to yield 0.01962 mol of CO2 and 0.01961 mol of H2O. What is the empirical formula of the compound? A) B) C) D) E) CHO C3H3O2 C2H2O C3H6O2 C6H3O2 ANS: D PTS: 1 DIF: moderate REF: 3.4 OBJ: Calculate the percentage of C, H, and O from combustion data. (Example 3.9) TOP: stoichiometry | determining chemical formulas Test Bank General Chemistry, 10th edition 20 79. A sample containing only carbon, hydrogen, phosphorus, and oxygen is subjected to elemental analysis. After complete combustion, a 0.4946-g sample of the compound yields 0.7092 g of CO2, 0.4355 g of H2O, and 0.3812 g of P4O10. What is the empirical formula of the compound? A) CH3PO B) C2H3PO C) C2H6P2O4 D) C3H9PO E) CH2P4O13 ANS: OBJ: TOP: MSC: D PTS: 1 DIF: difficult REF: 3.4 Calculate the percentage of C, H, and O from combustion data. (Example 3.9) stoichiometry | determining chemical formulas KEY: elemental analysis general chemistry 80. A sample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. After complete combustion, a 0.1099-g sample of the compound yields 0.2193 g of CO2, 0.1346 g of H2O, and 0.07485 g of SiO2. What is the empirical formula of the compound? A) CH3Si B) C2H4Si C) C4H12Si D) C6H12Si2 E) CH2Si ANS: OBJ: TOP: MSC: C PTS: 1 DIF: difficult REF: 3.4 Calculate the percentage of C, H, and O from combustion data. (Example 3.9) stoichiometry | determining chemical formulas KEY: elemental analysis general chemistry 81. Of the following, the only empirical formula is A) C4H10. B) C4H6. C) C5H14. D) H2O2. E) O2. ANS: OBJ: TOP: MSC: C PTS: 1 DIF: easy Define empirical formula. stoichiometry | determining chemical formulas general chemistry REF: 3.5 KEY: empirical formula 82. Which of the following is the empirical formula for the molecule below? A) B) C) D) CHO CH3COOH C2H4O2 CH2O Test Bank General Chemistry, 10th edition 21 E) none of the above. ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy Define empirical formula. stoichiometry | determining chemical formulas general chemistry REF: 3.5 KEY: empirical formula 83. Analysis of a compound showed that it contained 76.0 % fluorine atoms and 24.0 % carbon atoms by mass. What is its empirical formula? A) CF2 B) C2F3 C) CF3 D) C2F5 E) CF ANS: A PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the empirical formula of a binary compound from the masses of its elements. (Example 3.10) TOP: stoichiometry | determining chemical formulas KEY: empirical formula MSC: general chemistry 84. A sample of an oxide of antimony (Sb) contained 39.5 g of antimony combined with 13.0 g of oxygen. What is the simplest formula for the oxide? A) SbO2 B) SbO C) Sb2O3 D) Sb2O E) Sb2O5 ANS: E PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the empirical formula of a binary compound from the masses of its elements. (Example 3.10) TOP: stoichiometry | determining chemical formulas KEY: empirical formula MSC: general chemistry 85. Chlorine was passed over 1.30 g of heated titanium, and 4.20 g of a chloride-containing compound of Ti was obtained. What is the empirical formula of the chloride-containing compound? A) TiCl2 B) TiCl4 C) TiCl D) TiCl3 E) Ti2Cl3 ANS: D PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the empirical formula of a binary compound from the masses of its elements. (Example 3.10) TOP: stoichiometry | determining chemical formulas KEY: empirical formula MSC: general chemistry Test Bank General Chemistry, 10th edition 22 86. A 2.39-g sample of an oxide of chromium contains 1.48 g of chromium. Calculate the simplest formula for the compound. A) CrO5 B) Cr2O C) CrO2 D) CrO E) Cr2O3 ANS: C PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the empirical formula of a binary compound from the masses of its elements. (Example 3.10) TOP: stoichiometry | determining chemical formulas KEY: empirical formula MSC: general chemistry 87. A compound is composed of only C and H. It contains 92.26 % C. What is its empirical formula? A) C2H5 B) C2H3 C) C3H4 D) CH E) CH2 ANS: OBJ: TOP: MSC: D PTS: 1 DIF: easy REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 88. A compound composed of only C and F contains 17.39 % C by mass. What is its empirical formula? A) CF3 B) CF C) C2F D) CF4 E) CF2 ANS: OBJ: TOP: MSC: A PTS: 1 DIF: easy REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 89. A hydrocarbon, subjected to elemental analysis, was found to contain 85.63 % carbon and 14.37 % hydrogen by mass. What is the empirical formula of the hydrocarbon? A) CH4 B) C2H4 C) C6H D) C10H E) CH2 ANS: E PTS: 1 DIF: easy REF: 3.5 OBJ: Determine the empirical formula from the percentage composition. (Example 3.11) TOP: stoichiometry | determining chemical formulas KEY: empirical formula Test Bank General Chemistry, 10th edition 23 MSC: general chemistry 90. A particular compound contains, by mass, 41.4 % carbon, 3.47 % hydrogen, and 55.1 % oxygen. A 0.050-mol sample of this compound weighs 5.80 g. The molecular formula of this compound is A) C3H3O3. B) C3H3O. C) CHO. D) C4H4O4. E) C5H5O5. ANS: OBJ: TOP: KEY: D PTS: 1 DIF: moderate REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas molecular formula MSC: general chemistry 91. What is the empirical formula of an oxide of nitrogen that contains 25.93 % nitrogen by mass? A) NO2 B) N2O C) N2O3 D) NO E) N2O5 ANS: OBJ: TOP: MSC: E PTS: 1 DIF: moderate REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 92. The analysis of an organic compound showed that it contained 1.386 mol of C, 0.0660 mol of H, 0.924 mol of O, and 0.462 mol of N. How many nitrogen atoms are there in the empirical formula for this compound? A) 9 B) 7 C) 2 D) 4 E) 3 ANS: OBJ: TOP: MSC: B PTS: 1 DIF: easy REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 93. A sample containing 0.700 mol of a compound is composed of 4.21 1023 atoms of sodium, 24.79 g of chlorine atoms, and 33.57 g of oxygen atoms. The formula of the compound is A) NaClO3. B) NaClO5. C) NaClO. D) NaClO4. E) NaClO2. Test Bank General Chemistry, 10th edition 24 ANS: OBJ: TOP: MSC: A PTS: 1 DIF: moderate REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 94. The analysis of an organic compound showed that it contained 0.0700 mol of C, 0.175 mol of H, and 0.0350 mol of N. Its molecular mass is 86 amu. How many atoms of carbon are there in the empirical formula for the compound, and how many are in the molecular formula? A) empirical = 2, molecular = 3 B) empirical = 2, molecular = 6 C) empirical = 2, molecular = 4 D) empirical = 5, molecular = 10 E) empirical = 3, molecular = 3 ANS: OBJ: TOP: KEY: C PTS: 1 DIF: moderate REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas molecular formula MSC: general chemistry 95. A given hydrocarbon is burned in the presence of oxygen gas and is converted completely to water and carbon dioxide. The mole ratio of H2O to CO2 is 1.33:1.00. The hydrocarbon could be A) C2H2. B) C2H6. C) CH4. D) C3H4. E) C3H8. ANS: OBJ: TOP: MSC: E PTS: 1 DIF: difficult REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 96. A given hydrocarbon is burned in the presence of oxygen gas and is converted completely to carbon dioxide and water. Equal numbers of moles of CO2 and H2O are produced. The hydrocarbon could be A) C3H4. B) C5H10. C) C2H3. D) CH3. E) C3H5. ANS: OBJ: TOP: MSC: B PTS: 1 DIF: difficult REF: 3.5 Determine the empirical formula from the percentage composition. (Example 3.11) stoichiometry | determining chemical formulas KEY: empirical formula general chemistry 97. An organic compound that has the empirical formula CHO has a molecular mass of 145 amu. Its molecular formula is Test Bank General Chemistry, 10th edition 25 A) B) C) D) E) C9H9O9. C4H4O4. C3H3O3. C5H5O5. C12H12O12. ANS: D PTS: 1 DIF: easy REF: 3.5 OBJ: Understand the relationship between the molecular mass of a substance and its empirical formula mass. TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 98. A certain compound has a molar mass of 210 g/mol. Which is a possible empirical formula for this compound? A) CH2O B) CHO C) C2H2O2 D) C2HO E) C2H2O ANS: A PTS: 1 DIF: moderate REF: 3.5 OBJ: Understand the relationship between the molecular mass of a substance and its empirical formula mass. TOP: stoichiometry | determining chemical formulas KEY: empirical formula MSC: general chemistry 99. The empirical formula for a group of compounds is CHCl. Lindane, a powerful insecticide, is a member of this group. The molar mass of lindane is 290.8. How many atoms of carbon does a molecule of lindane contain? A) 3 B) 2 C) 4 D) 6 E) 8 ANS: D PTS: 1 DIF: easy REF: 3.5 OBJ: Understand the relationship between the molecular mass of a substance and its empirical formula mass. TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 100. The empirical formula of styrene is CH; its molar mass is 104.1 g/mol. What is the molecular formula of styrene? A) C6H6 B) C2H4 C) C8H8 D) C10H12 E) none of these ANS: C PTS: 1 DIF: easy REF: 3.5 OBJ: Understand the relationship between the molecular mass of a substance and its Test Bank General Chemistry, 10th edition 26 empirical formula mass. TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 101. The empirical formula of styrene is CH. An experimental determination of the molar mass of styrene by a student yields the value of 104 g/mol. What is the molecular formula of styrene? A) C5H10 B) CH C) C8H8 D) C3H8 E) C6H9 ANS: C PTS: 1 DIF: easy REF: 3.5 OBJ: Understand the relationship between the molecular mass of a substance and its empirical formula mass. TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 102. An organic compound has a molecular mass of 279.2 amu and contains 85.96% carbon by mass. How many carbon atoms are in each molecule of this compound? A) 16 B) 21 C) 25 D) 29 E) 20 ANS: E PTS: 1 DIF: easy REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 103. An unknown organic compound contains 41.4 % carbon, 3.47 % hydrogen, and 55.1 % oxygen by mass. A 0.040-mol sample of this compound weighs 3.48 g. What is the molecular formula of the organic compound? A) C3H3O B) C2H2O2 C) C3H3O3 D) C7H7O7 E) CHO ANS: C PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 104. A compound is composed of only C and I. It contains 5.935 % C by mass and has a molar mass of 809.44 g/mol. What is its molecular formula? Test Bank General Chemistry, 10th edition 27 A) B) C) D) E) CI C4I6 C3I4 C2I2 C2I3 ANS: B PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 105. An organic compound has a molar mass of 171.1 g/mol and contains 11.10 % hydrogen atoms by mass. How many hydrogen atoms are in each molecule of this compound? A) 19 B) 6 C) 21 D) 28 E) 11 ANS: A PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 106. A compound has a molar mass of 171.6 g/mol and contains 55.94 % oxygen atoms by mass. How many oxygen atoms are in each molecule of this compound? A) 6 B) 4 C) 10 D) 2 E) 8 ANS: A PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 107. A compound contains 43.84 % carbon atoms, 3.65 % hydrogen atoms, and 8.68 % fluorine atoms by mass. Each molecule of this compound contains one fluorine atom. What is the total number of carbon, hydrogen, and fluorine atoms in one molecule of this compound? A) 17 B) 12 C) 9 D) 7 E) 14 ANS: A PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular Test Bank General Chemistry, 10th edition 28 mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 108. A molecular compound contains 92.3 % carbon and 7.7 % hydrogen by mass. If 0.432 mol of the compound weighs 22.46 g, what is its molecular formula? A) C8H8 B) C6H10 C) C4H8 D) C4H4 E) CH ANS: D PTS: 1 DIF: moderate REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 109. Analysis of a compound containing only C and Br revealed that it contains 33.33 % C atoms by number and has a molar mass of 515.46 g/mol. What is the molecular formula of this compound? A) CBr2 B) C2Br6 C) C2Br4 D) CBr3 E) C3Br6 ANS: E PTS: 1 DIF: difficult REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 110. Complete combustion of a 0.30-mol sample of a hydrocarbon, CxHy, gives 1.20 mol of CO2 and 1.50 mol of H2O. The molecular formula of the original hydrocarbon is A) C3H8. B) C4H10. C) C8H20. D) C3H5. E) C5H7. ANS: B PTS: 1 DIF: difficult REF: 3.5 OBJ: Determine the molecular formula from the percentage composition and molecular mass. (Example 3.12) TOP: stoichiometry | determining chemical formulas KEY: molecular formula MSC: general chemistry 111. A chemical reaction has the equation: 2A + B C. Which of the following figures best illustrates a stoichiometric ratio of A and B? Test Bank General Chemistry, 10th edition 29 I. A) B) C) D) E) II. III. IV. I only III only II only Both I and IV IV only ANS: D PTS: 1 DIF: easy REF: 3.6 OBJ: Relate the coefficients in a balanced chemical equation to the number of molecules or moles (molar interpretation). TOP: stoichiometry | stoichiometry calculation KEY: molar interpretation MSC: general chemistry 112. The balanced chemical equation for the combustion of methane is: CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) Which of the following statements concerning this chemical equation is/are correct? 1. 2. 3. A) B) C) D) E) One gram of methane gas reacts with two grams of dioxygen gas, producing one gram of carbon dioxide gas and two grams of gaseous water. One mole of methane gas reacts with two moles of dioxygen gas, producing one mole of carbon dioxide gas and two moles of gaseous water. One molecule of methane gas reacts with two molecules of dioxygen gas, producing one molecule of carbon dioxide gas and two molecules of gaseous water. 1 only 2 only 2 and 3 1 and 3 1,2 and 3 ANS: C PTS: 1 DIF: easy REF: 3.6 OBJ: Relate the coefficients in a balanced chemical equation to the number of molecules or moles (molar interpretation). TOP: stoichiometry | stoichiometry calculation KEY: molar interpretation MSC: general chemistry NOT: REVISED 113. Balance the following expression: __ CH3CH2COOH + __ O2 __ CO2 + __ H2O How many moles of O2 are required for the complete combustion of 8 mol of propanoic acid? A) 5 mol B) 30 mol Test Bank General Chemistry, 10th edition 30 C) 28 mol D) 37 mol E) 2 mol ANS: C PTS: 1 DIF: easy REF: 3.6 OBJ: Relate the coefficients in a balanced chemical equation to the number of molecules or moles (molar interpretation). TOP: stoichiometry | stoichiometry calculation KEY: molar interpretation MSC: general chemistry 114. The products of the combustion of acetone with oxygen are shown in the following equation: __ CH3COCH3 + __ O2 __ CO2 + __ H2O When properly balanced, the equation indicates that ____ mol of CO2 are produced for each mole of CH3COCH3. A) 3 B) 5 C) 4.5 D) 8 E) 1 ANS: A PTS: 1 DIF: easy REF: 3.6 OBJ: Relate the coefficients in a balanced chemical equation to the number of molecules or moles (molar interpretation). TOP: stoichiometry | stoichiometry calculation KEY: molar interpretation MSC: general chemistry 115. Ammonia, NH3, and oxygen can be reacted together in the presence of a catalyst to form only nitrogen monoxide and water. The number of moles of oxygen consumed for every 5.00 moles of NO produced is . A) 6.25 B) 25.0 C) 18.8 D) 3.13 E) 12.5 ANS: A PTS: 1 DIF: difficult REF: 3.6 OBJ: Relate the coefficients in a balanced chemical equation to the number of molecules or moles (molar interpretation). TOP: stoichiometry | stoichiometry calculation KEY: molar interpretation MSC: general chemistry 116. 2KHCO3(s) K2CO3(s) + CO2(g) + H2O(l) How many moles of potassium carbonate will be produced if 454 g of potassium hydrogen carbonate are heated? A) 2.27 mol B) 3.29 mol C) 11.4 mol D) 227 mol Test Bank General Chemistry, 10th edition 31 E) 4.54 mol ANS: OBJ: TOP: KEY: A PTS: 1 DIF: moderate REF: 3.7 Relate the quantities of reactant to the quantity of product. (Example 3.13) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry 117. Calculate the number of moles of O2 required to react with phosphorus to produce 4.76 g of P4O6. (Molar mass P4O6 = 219.9 g/mol) A) 0.0216 mol B) 0.149 mol C) 0.0649 mol D) 0.0433 mol E) 0.130 mol ANS: OBJ: TOP: KEY: C PTS: 1 DIF: moderate REF: 3.7 Relate the quantities of reactant to the quantity of product. (Example 3.13) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry 118. Elemental sulfur can be converted to sulfur dioxide by combustion in air. Sulfur dioxide will react with water to form sulfurous acid (see balanced equation below). SO2(g) + H2O(l) H2SO3(l) What mass of sulfur dioxide is needed to prepare 36.86 g of H2SO3(l)? A) B) C) D) E) 28.77 g 47.23 g 0.5754 g 0.4491 g 36.86 g ANS: A PTS: 1 DIF: moderate REF: 3.7 OBJ: Relate the quantities of reactant to the quantity of product. (Example 3.13) TOP: stoichiometry | stoichiometry calculation 119. One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(III) hydroxide from a solution containing rhodium(III) sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq) + 6NaOH(aq) 2Rh(OH)3(s) + 3Na2SO4(aq) If 2.40 g of rhodium(III) sulfate reacts with excess sodium hydroxide, what mass of rhodium(III) hydroxide may be produced? A) 1.50 g B) 4.80 g C) 2.40 g D) 0.374 g E) 2.99 g ANS: A Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.7 32 OBJ: Relate the quantities of reactant to the quantity of product. (Example 3.13) TOP: stoichiometry | stoichiometry calculation KEY: amounts of substances MSC: general chemistry 120. Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) What mass of copper(I) sulfide is required in order to prepare 0.610 kg of copper metal? A) 0.610 kg B) 0.305 kg C) 0.459 kg D) 1.53 kg E) 0.764 kg ANS: OBJ: TOP: KEY: E PTS: 1 DIF: easy REF: 3.7 Relate the quantities of reactant to the quantity of product. (Example 3.13) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry 121. One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(III) hydroxide from a solution containing rhodium(III) sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq) + 6NaOH(aq) 2Rh(OH)3(s) + 3Na2SO4(aq) What mass of sodium hydroxide is required to precipitate 74.0 g of rhodium(III) hydroxide from a solution containing excess rhodium(III) sulfate? A) 6.41 g B) 57.7 g C) 19.2 g D) 222 g E) 74.0 g ANS: OBJ: TOP: KEY: B PTS: 1 DIF: easy REF: 3.7 Relate the quantities of reactant to the quantity of product. (Example 3.13) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry 122. Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) If 0.680 kg of copper(I) sulfide reacts with excess oxygen, what mass of copper metal may be produced? A) 0.680 kg B) 0.136 kg C) 0.271 kg D) 0.543 kg E) 1.36 kg ANS: D Test Bank PTS: 1 DIF: easy General Chemistry, 10th edition REF: 3.7 33 OBJ: Relate the quantities of two reactants or two products. (Example 3.14) TOP: stoichiometry | stoichiometry calculation KEY: amounts of substances MSC: general chemistry 123. One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(III) hydroxide from a solution containing rhodium(III) sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq) + 6NaOH(aq) 2Rh(OH)3(s) + 3Na2SO4(aq) If 0.620 g of rhodium(III) hydroxide is produced, what mass of sodium sulfate is also produced? A) 0.572 g B) 0.930 g C) 0.858 g D) 0.620 g E) 0.381 g ANS: OBJ: TOP: KEY: C PTS: 1 DIF: easy REF: 3.7 Relate the quantities of two reactants or two products. (Example 3.14) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry 124. The balanced equation for the combustion of ethanol is 2C2H5OH(g) + 7O2(g) 4CO2(g) + 6H2O(g) How many grams of dioxygen are required to burn 5.9 g of C2H5OH? A) B) C) D) E) 14 g 21 g 4.1 g 38 g 55 g ANS: A PTS: 1 DIF: moderate REF: 3.7 OBJ: Relate the quantities of two reactants or two products. (Example 3.14) TOP: stoichiometry | stoichiometry calculation 125. 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2(g) According to the equation above, how many grams of aluminum are needed to completely react with 3.83 mol of hydrochloric acid? A) 310 g B) 46.6 g C) 34.4 g D) 3.83 g E) 103.3 g ANS: OBJ: TOP: KEY: C PTS: 1 DIF: moderate REF: 3.7 Relate the quantities of two reactants or two products. (Example 3.14) stoichiometry | stoichiometry calculation amounts of substances MSC: general chemistry Test Bank General Chemistry, 10th edition 34 126. A chemical reaction has the equation: 2A + B C. In which case is B the limiting reactant? A) B) C) D) E) II I III IV none of these ANS: A PTS: 1 DIF: easy REF: 3.8 OBJ: Understand how a limiting reactant or limiting reagent determines the moles of product formed during a chemical reaction and how much excess reactant remains. TOP: stoichiometry | stoichiometry calculation KEY: limiting reactant MSC: general chemistry 127. Consider an initial mixture of CH4 and O2 represented in the container below: Given the reaction CH4 + 2O2 CO2 + 2H2O, which of the following represents a stoichiometric picture of the container after the reaction has gone to completion? A) Test Bank General Chemistry, 10th edition 35 B) C) D) E) none of the above ANS: C PTS: 1 DIF: easy REF: 3.8 OBJ: Understand how a limiting reactant or limiting reagent determines the moles of product formed during a chemical reaction and how much excess reactant remains. TOP: stoichiometry | stoichiometry calculation KEY: limiting reactant MSC: general chemistry 128. Which of the following statements concerning the limiting reactant is/are correct? 1. 2. 3. A) B) C) D) E) The mass of the limiting reactant is the always the lowest mass of all reactant masses. The theoretical yield depends on the amount of limiting reactant. The moles of limiting reactant is always the lowest moles of all reactants. 2 only 3 only 1 and 3 2 and 3 1, 2, and 3 ANS: A PTS: 1 DIF: moderate REF: 3.8 OBJ: Understand how a limiting reactant or limiting reagent determines the moles of product formed during a chemical reaction and how much excess reactant remains. TOP: stoichiometry | stoichiometry calculation 129. The limiting reactant is the reactant Test Bank General Chemistry, 10th edition 36 A) B) C) D) E) that has the lowest coefficient in the balanced equation. that has the lowest molar mass. that is left over after the reaction has gone to completion. for which there is the lowest mass in grams. none of the above ANS: E PTS: 1 DIF: easy REF: 3.8 OBJ: Understand how a limiting reactant or limiting reagent determines the moles of product formed during a chemical reaction and how much excess reactant remains. TOP: stoichiometry | stoichiometry calculation KEY: limiting reactant MSC: general chemistry 130. The commercial production of phosphoric acid, H3PO4, can be represented by the equation 1500 g 300 g 307 g Ca3(PO4)2 + 3SiO2 + 5C + 1180 g 5O2 + 300 g 3H2O 310 g/mol 32.0 g/mol 18.0 g/mol 60.1 g/mol 12.0 g/mol 3CaSiO3 + 5CO2 + 2H3PO4 The molar mass for each reactant is shown below the reactant, and the mass of each reactant for this problem is given above. Which substance is the limiting reactant? A) H2O B) C C) O2 D) Ca3(PO4)2 E) SiO2 ANS: OBJ: TOP: MSC: E PTS: 1 DIF: moderate REF: 3.8 Calculate with a limiting reactant involving masses. (Example 3.16) stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry 131. SO2 reacts with H2S as follows: 2H2S + SO2 3S + 2H2O When 7.50 g of H2S reacts with 12.75 g of SO2, which statement applies? A) 6.38 g of sulfur is formed. B) SO2 is the limiting reagent. C) 0.0216 mol of H2S remains. D) 10.6 g of sulfur is formed. E) 1.13 g of H2S remains. ANS: OBJ: TOP: MSC: D PTS: 1 DIF: moderate REF: 3.8 Calculate with a limiting reactant involving masses. (Example 3.16) stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry 132. A 15-g sample of lithium is reacted with 15 g of fluorine to form lithium fluoride: 2Li + F2 2LiF. After the reaction is complete, what will be present? A) 0.789 mol of lithium fluoride only B) 2.16 mol of lithium fluoride only C) 2.16 mol of lithium fluoride and 0.395 mol of fluorine Test Bank General Chemistry, 10th edition 37 D) 0.789 mol of lithium fluoride and 1.37 mol of lithium E) none of these ANS: OBJ: TOP: MSC: D PTS: 1 DIF: difficult REF: 3.8 Calculate with a limiting reactant involving masses. (Example 3.16) stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry 133. When 20.0 g C2H6 and 60.0 g O2 react to form CO2 and H2O, how many grams of water are formed? A) 14.5 g B) 58.0 g C) 18.0 g D) 20.0 g E) none of these ANS: OBJ: TOP: MSC: E PTS: 1 DIF: moderate REF: 3.8 Calculate with a limiting reactant involving masses. (Example 3.16) stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry 134. If 48.8 g of O2 is mixed with 48.8 g of H2 and the mixture is ignited, what is the maximum mass of water that may be produced? A) 439 g B) 54.9 g C) 48.8 g D) 98 g E) 86.8 g ANS: OBJ: TOP: MSC: B PTS: 1 DIF: moderate REF: 3.8 Define and calculate the theoretical yield of chemical reactions. stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry 135. One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(III) hydroxide from a solution containing rhodium(III) sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq) + 6NaOH(aq) 2Rh(OH)3(s) + 3Na2SO4(aq) What is the theoretical yield of rhodium(III) hydroxide from the reaction of 0.590 g of rhodium(III) sulfate with 0.266 g of sodium hydroxide? A) 0.341 g B) 0.266 g C) 0.184 g D) 0.856 g E) 0.368 g ANS: OBJ: TOP: MSC: A PTS: 1 DIF: moderate REF: 3.8 Define and calculate the theoretical yield of chemical reactions. stoichiometry | stoichiometry calculation KEY: limiting reactant general chemistry Test Bank General Chemistry, 10th edition 38 136. A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation 2AgNO3(aq) + BaCl2(aq) 2AgCl(s) + Ba(NO3)2(aq) to give 3.36 g of AgCl. What is the percent yield of AgCl? A) 44.6 % B) 33.5 % C) 66.9 % D) 56.5 % E) 100 % ANS: C PTS: 1 DIF: moderate OBJ: Determine the percentage yield of a chemical reaction. TOP: stoichiometry | stoichiometry calculation REF: 3.8 MSC: general chemistry 137. The reaction of 11.9 g of CHCl3 with excess chlorine produced 10.2 g of CCl4, carbon tetrachloride: 2CHCl3 + 2Cl2 2CCl4 + 2HCl What is the percent yield? A) 85.7 % B) 100 % C) 66.5 % D) 33.3 % E) 44.3 % ANS: C PTS: 1 DIF: moderate OBJ: Determine the percentage yield of a chemical reaction. TOP: stoichiometry | stoichiometry calculation REF: 3.8 MSC: general chemistry 138. Consider the following reaction: 2A + B 3C + D 3.0 mol A and 2.0 mol B react to form 4.0 mol C. What is the percent yield of this reaction? A) 75 % B) 67 % C) 89 % D) 50 % E) 100 % ANS: OBJ: TOP: MSC: C PTS: 1 DIF: moderate Determine the percentage yield of a chemical reaction. stoichiometry | stoichiometry calculation general chemistry REF: 3.8 KEY: limiting reactant 139. Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) If the reaction of 0.630 kg of copper(I) sulfide with excess oxygen produces 0.190 kg of copper metal, what is the percent yield? Test Bank General Chemistry, 10th edition 39 A) B) C) D) E) 75.5 % 39.9 % 30.2 % 151 % 37.8 % ANS: OBJ: TOP: MSC: E PTS: 1 DIF: difficult Determine the percentage yield of a chemical reaction. stoichiometry | stoichiometry calculation general chemistry REF: 3.8 KEY: limiting reactant 140. One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(III) hydroxide from a solution containing rhodium(III) sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq) + 6NaOH(aq) 2Rh(OH)3(s) + 3Na2SO4(aq) If the reaction of 0.650 g of rhodium(III) sulfate with excess sodium hydroxide produces 0.320 g of rhodium(III) hydroxide, what is the percent yield? A) 316 % B) 158 % C) 39.5 % D) 49.2 % E) 79.0 % ANS: OBJ: TOP: MSC: E PTS: 1 DIF: difficult Determine the percentage yield of a chemical reaction. stoichiometry | stoichiometry calculation general chemistry REF: 3.8 KEY: limiting reactant 141. Sulfur trioxide, SO3, is made from the oxidation of SO2, and the reaction is represented by the equation: 2SO2 + O2 2SO3 A 21-g sample of SO2 gives 18 g of SO3. The percent yield of SO3 is A) 11 % B) 69 % C) 17 % D) 26 % E) 100 % ANS: B PTS: 1 DIF: moderate OBJ: Determine the percentage yield of a chemical reaction. TOP: stoichiometry | stoichiometry calculation . REF: 3.8 MSC: general chemistry 142. Nitric oxide, NO, is made from the oxidation of NH3, and the reaction is represented by the equation 4NH3 + 5O2 4NO + 6H2O An 9.1-g sample of NH3 gives 12.0 g of NO. The percent yield of NO is A) 94 % B) 46 % Test Bank General Chemistry, 10th edition . 40 C) 17 % D) 75 % E) 28 % ANS: D PTS: 1 DIF: moderate OBJ: Determine the percentage yield of a chemical reaction. TOP: stoichiometry | stoichiometry calculation REF: 3.8 MSC: general chemistry 143. Consider the fermentation reaction of glucose: C6H12O6 2C2H5OH + 2CO2 A 1.00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast. If 67.8 g of C2H5OH was obtained, what was the percent yield of C2H5OH? A) 73.6 % B) 36.8 % C) 67.8 % D) 100 % E) none of these ANS: OBJ: TOP: MSC: A PTS: 1 DIF: difficult Determine the percentage yield of a chemical reaction. stoichiometry | stoichiometry calculation general chemistry REF: 3.8 KEY: limiting reactant 144. One commercial system removes SO2 emissions from smoke at 95.0°C by the following set of balanced reactions: SO2(g) + Cl2 SO2Cl2(g) SO2Cl2 + 2H2O H2SO4 + 2HCl H2SO4 + Ca(OH)2 CaSO4(s) + 2H2O Assuming the process is 95.0 % efficient, how many grams of CaSO4 may be produced from 100. g of SO2? (molar masses: SO2, 64.1 g/mol; CaSO4, 136 g/mol) A) 87.2 g B) 202 g C) 44.8 g D) 47.1 g E) 212 g ANS: OBJ: TOP: MSC: B PTS: 1 DIF: difficult Determine the percentage yield of a chemical reaction. stoichiometry | stoichiometry calculation general chemistry Test Bank General Chemistry, 10th edition REF: 3.8 KEY: limiting reactant 41