quiz2-0708ans
advertisement
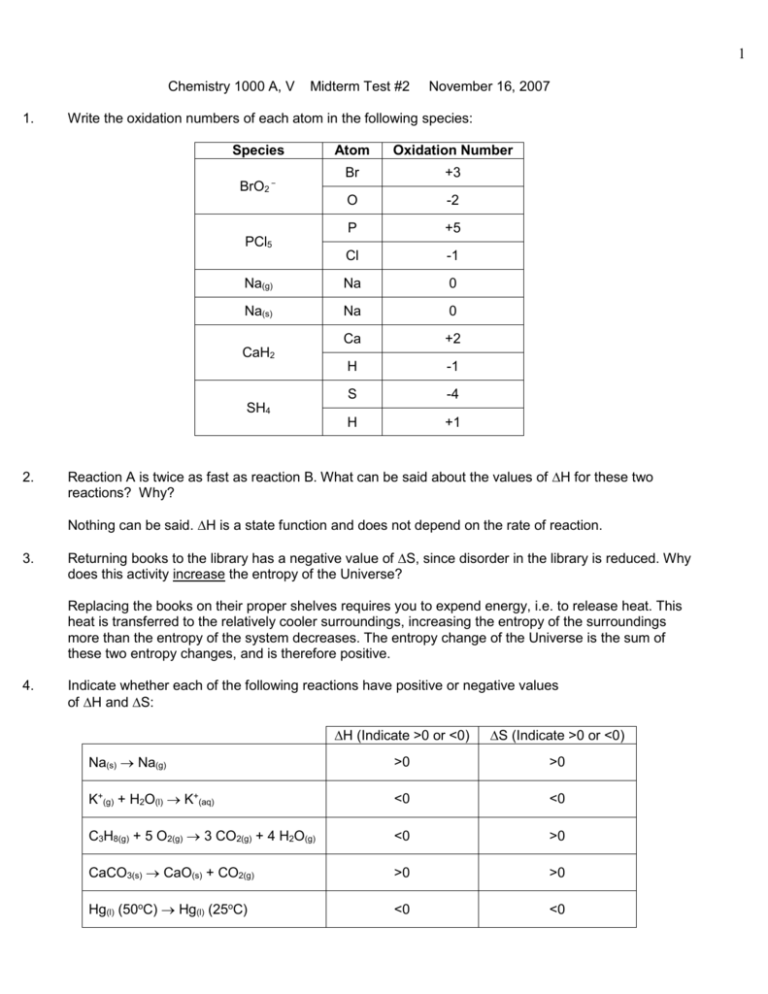
1 Chemistry 1000 A, V 1. Midterm Test #2 November 16, 2007 Write the oxidation numbers of each atom in the following species: Species Atom Oxidation Number Br +3 O -2 P +5 Cl -1 Na(g) Na 0 Na(s) Na 0 Ca +2 H -1 S -4 H +1 BrO2‾ PCl5 CaH2 SH4 2. Reaction A is twice as fast as reaction B. What can be said about the values of H for these two reactions? Why? Nothing can be said. H is a state function and does not depend on the rate of reaction. 3. Returning books to the library has a negative value of S, since disorder in the library is reduced. Why does this activity increase the entropy of the Universe? Replacing the books on their proper shelves requires you to expend energy, i.e. to release heat. This heat is transferred to the relatively cooler surroundings, increasing the entropy of the surroundings more than the entropy of the system decreases. The entropy change of the Universe is the sum of these two entropy changes, and is therefore positive. 4. Indicate whether each of the following reactions have positive or negative values of H and S: H (Indicate >0 or <0) S (Indicate >0 or <0) Na(s) Na(g) >0 >0 K+(g) + H2O(l) K+(aq) <0 <0 C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) <0 >0 CaCO3(s) CaO(s) + CO2(g) >0 >0 Hg(l) (50oC) Hg(l) (25oC) <0 <0 2 5. For the reaction PCl3(g) + Cl2(g) Ý PCl5(g) (for which H < 0) initially at equilibrium, which direction will the equilibrium shift (left or right) if we make the following changes? Explain why in each case. (a) Add some PCl5(g) The reaction will shift to the LEFT in order for the concentration of PCl5(g) to decrease. (b) Lower the temperature The reaction will shift to the RIGHT, since this is the exothermic direction. 6. The bond energy of a particular bond is observed to decrease when the molecule is positively ionized. What can be said about the molecular orbital from which the electron was removed? Why? The electron must have been removed from a bonding molecular orbital, since the bond order must have decreased. B1. (a) (16 marks) Balance the following REDOX reaction in acidic solution: Cr+3(aq) + NO3‾(aq) Cr2O7-2(aq) + NO2(g) Oxidation: Cr+3(aq) Cr2O7-2(aq) 2 Cr+3(aq) Cr2O7-2(aq) 2 Cr+3(aq) + 7 H2O(l) Cr2O7-2(aq) 2 Cr+3(aq) + 7 H2O(l) Cr2O7-2(aq) 14 H+(aq) 2 Cr+3(aq) + 7 H2O(l) Cr2O7-2(aq) 14 H+(aq) + 6 eReduction: NO3‾(aq) NO2(g) NO3‾(aq) NO2(g) + H2O(l) NO3‾(aq) + 2 H+(aq) NO2(g) + H2O(l) NO3‾(aq) + 2 H+(aq) + e- NO2(g) + H2O(l) Overall: 2 Cr+3(aq) + 7 H2O(l) Cr2O7-2(aq) 14 H+(aq) + 6 e6 NO3‾(aq) + 12 H+(aq) + 6 e- 6 NO2(g) + 6 H2O(l) __________________________________________________ 2 Cr+3(aq) + H2O(l) + 6 NO3‾(aq) Cr2O7-2(aq) + 2 H+(aq) + 6 NO2(g) (b) (4 marks) Identify the oxidant and the reducing agent in the above REDOX reaction. NO3- gets reduced, so it must be the oxidant. (“N atom” is also an acceptable answer) Cr+3 gets oxidized, so it must be the reducing agent 3 B2. Referring to the MO diagram of B2, below, answer the following questions: (a) (4 marks) What is the bond order of B2? Bond Order 64 1 2 (b) (4 marks) Is the molecule paramagnetic? How do you know? Yes, it is paramagnetic because of the unpaired electrons in the 2p molecular orbital. (c) (4 marks) Which has the longer bond length: B2+ or B2? How do you know? The bond order of B2+ would be (5-4)/2 = 0.5 The bond order of B2 is 1 Since B2+ has the lower bond order, it has the longer bond length. (d) (4 marks) Which has the greater bond energy: B2‾ or B2? How do you know? The bond order of B2- would be (7-4)/2 = 1.5 The bond order of B2 is 1 Since B2‾ has the higher bond order, it has the greater bond energy. (e) (4 marks) Can B2+2 exist? Why or why not? To make B2+2 would require removing both electrons in the highest energy MO, i.e. the 2p. B2+2 would have a bond order of (4-4)/2 = 0, and so can not exist. B3. Given the reaction CaCO3(s) ∏ CaO(s) + CO2(g) and the thermodynamic data: CaCO3(s) CaO(s) CO2(g) ΔGfo (kJ/mol) -1128.8 -603.5 -394.4 So (J K-1 mol-1) 92.9 38.2 213.7 4 (a) (4 marks) Calculate the value of ΔG for the reaction at 25 C (in kJ mol ). o o -1 ΔGo = ΔGfo(CaO(s)) + ΔGfo(CO2(g)) - ΔGfo(CaCO3(s)) = -603.5 + (-394.4) – (-1128.8) = +130.9 kJ mol-1 (b) (2marks) Is this reaction spontaneous at 25oC? How do you know? This reaction is not spontaneous since ΔGo > 0. (c) (4 marks) Calculate the value of ΔSo for the reaction (in J K-1 mol-1). ΔSo = So(CaO(s)) + So(CO2(g)) - So(CaCO3(s)) = 38.2 + 213.7 – 92.9 = +159 J K-1 mol-1 (d) (4 marks) Calculate the value of ΔHo for the reaction (in kJ mol-1) at 25oC. ΔGo = ΔHo - TΔSo Thus, ΔHo = ΔGo + TΔSo = 130900 J mol-1 + (25+273.15) K (159 J K-1 mol-1) = 178305 J mol-1 = 178.3 kJ mol-1 (e) (6 marks) Calculate the temperature (in oC) above which the reaction is spontaneous. ΔG = ΔHo - TΔSo At this temperature, ΔG = 0. Thus, T Ho 178300Jmol1 1121K 848o C So 159JK 1mol1






