The Modern Periodic Table
advertisement

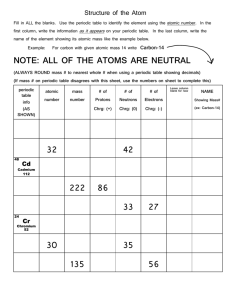
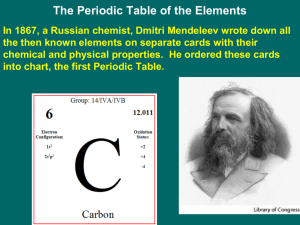
CHEMISTRY CHAPTER 6 NOTES MRS. SINGH SXN 6.1 Development of the Modern Periodic Table In _______, the Russian chemist Dmitri Mendeleev noted that when the known elements were placed in order of increasing atomic _________, their properties repeated in a regular _____________, a periodic pattern. Mendeleev made a table in which her arranged the _______________ in order of increasing atomic mass into ______________ with _________________ properties. From this table, Mendeleev predicted the properties of three elements that were undiscovered at the time. When these elements – scandium ( ), gallium ( ) , and germanium ( ) were soon discovered and found to have most of the _______________ properties, Mendeleev’s periodic table was _________________ in the scientific world. Besides the addition of newly discovered elements, the only ___________ change in the organization of the periodic table since Mendeleev’s time is that the elements are now arranged in order of atomic _______________ instead of atomic ___________. The Modern Periodic Table The elements are arranged in the periodic table in order of ____________________ atomic number in horizontal rows called _______________. Because the pattern of properties repeats in each new row of elements, the elements in a column have ______________ properties and are called a ____________ or family of elements. The groups are designated with a ___________ and the letter ____ or _____. 1 Groups 1A through 8A are called the main group or representative __________. The Group B elements are called the ________________ elements. Elements are divided into three main classes - _____________, metalloids, and ______________. Metals are generally __________ solids and are good _______________ of heat and electrical ______________. Some groups of elements have names. For examples, the first two groups of metals are called the _____________ metals and the alkaline ___________ metals. Most of the elements to the right of the heavy stair-step line in the periodic table are _________________, which are generally either ______ or brittle ____________ at room temperature. Group 7A elements are commonly called ______________, and group 8A elements are the ___________ gases. Many of the elements that border the stair-step line are metalloids, which have some of the characteristics of __________ metals and nonmetals. Practice Match each element in Column A with the best matching description from Column B. Column A element may match more than one description from Column B. 1. 2. 3. 4. 5. 6. 7. Column A strontium chromium iodine nitrogen argon rubidium silicon Column B a. halogen b. noble gas c. alkaline earth metal d. metalloid e. alkali metal f. representative element g. transition element 2 HOW TO WRITE A FORMULA 1. Write down the chemical symbols for each atom or element – the metal first then the nonmetal: Ex. Aluminum oxide Al O Calcium oxide Ca O 2. Write the valence/charge above each symbol. Al +3 O -2 Ca +2 O -2 3. Now criss-cross and reduce if possible Al +3 Al2O3 O –2 Ca +2 O –2 Ca2O2 reduces to CaO 3









![[Outline] Dmitri Mendeleev Presentation](http://s2.studylib.net/store/data/009977361_1-db3b9e6331e053f2ba93e6cb4861e0cd-300x300.png)