Grade 12 University Chemistry - Rosedale Heights School of the Arts
advertisement
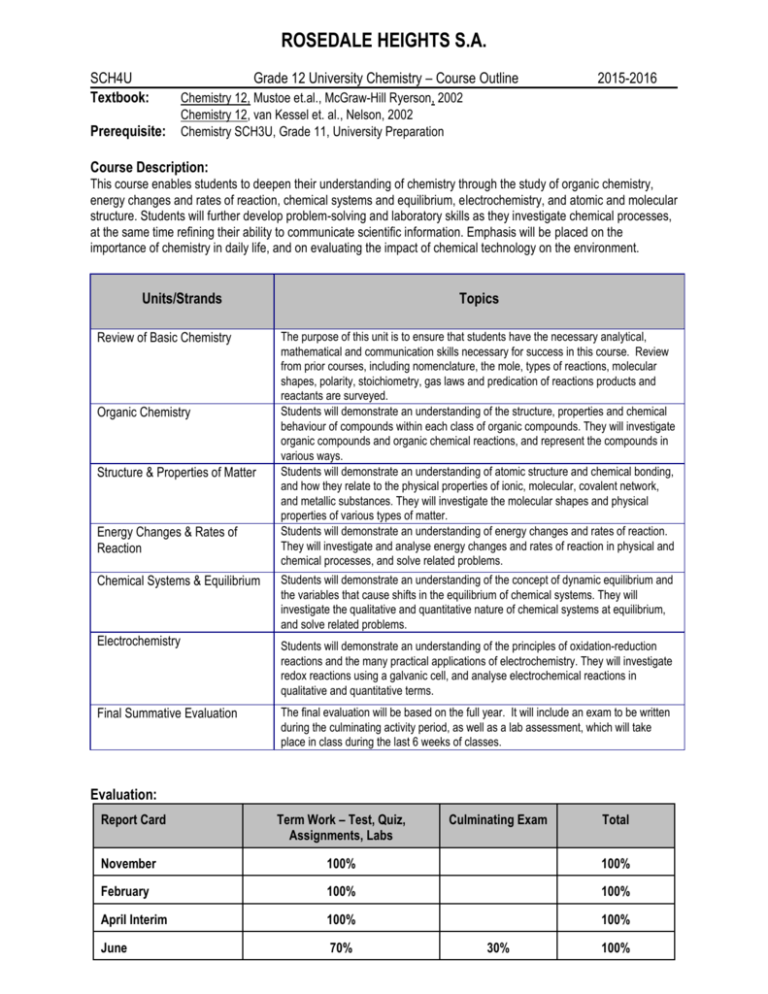
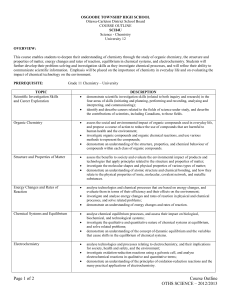
ROSEDALE HEIGHTS S.A. SCH4U Textbook: Prerequisite: Grade 12 University Chemistry – Course Outline 2015-2016 Chemistry 12, Mustoe et.al., McGraw-Hill Ryerson, 2002 Chemistry 12, van Kessel et. al., Nelson, 2002 Chemistry SCH3U, Grade 11, University Preparation Course Description: This course enables students to deepen their understanding of chemistry through the study of organic chemistry, energy changes and rates of reaction, chemical systems and equilibrium, electrochemistry, and atomic and molecular structure. Students will further develop problem-solving and laboratory skills as they investigate chemical processes, at the same time refining their ability to communicate scientific information. Emphasis will be placed on the importance of chemistry in daily life, and on evaluating the impact of chemical technology on the environment. Units/Strands Review of Basic Chemistry Organic Chemistry Structure & Properties of Matter Energy Changes & Rates of Reaction Chemical Systems & Equilibrium Electrochemistry Final Summative Evaluation Topics The purpose of this unit is to ensure that students have the necessary analytical, mathematical and communication skills necessary for success in this course. Review from prior courses, including nomenclature, the mole, types of reactions, molecular shapes, polarity, stoichiometry, gas laws and predication of reactions products and reactants are surveyed. Students will demonstrate an understanding of the structure, properties and chemical behaviour of compounds within each class of organic compounds. They will investigate organic compounds and organic chemical reactions, and represent the compounds in various ways. Students will demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. They will investigate the molecular shapes and physical properties of various types of matter. Students will demonstrate an understanding of energy changes and rates of reaction. They will investigate and analyse energy changes and rates of reaction in physical and chemical processes, and solve related problems. Students will demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. They will investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems. Students will demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry. They will investigate redox reactions using a galvanic cell, and analyse electrochemical reactions in qualitative and quantitative terms. The final evaluation will be based on the full year. It will include an exam to be written during the culminating activity period, as well as a lab assessment, which will take place in class during the last 6 weeks of classes. Evaluation: Report Card Term Work – Test, Quiz, Assignments, Labs Culminating Exam Total November 100% 100% February 100% 100% April Interim 100% 100% June 70% 30% 100% Term work includes all labs, assignments, group work, quizzes and unit tests. The four categories of Knowledge and Understanding, Thinking and Investigation, Communication, and Application encompasses the curriculum expectations of all high school courses. Teaching assessment, evaluation, feedback and the final grade will be based on these four categories with a minimum weighting of 20% per category. Student Responsibilities: Students are expected to be present for all classes and to complete homework on a regular basis. It is the student’s responsibility to follow the appropriate lab protocol and safety procedures. Students are responsible for all material covered in class during their absence. It is the student’s responsibility to inform the teacher of any planned absence, and arrange with the teacher to make up all missed work. Failing to communicate with your teacher the reason for your absence will result in a zero for any assessments during the absence. Seek extra help, it is available. Listed below is an outline of the units of study in 12 University Chemistry and the topics covered within each unit. The amount of time spent on each topic will vary, and the order may change. TERM 1: UNIT TOPICS 1. Mole Review · · · · Mol calculations Stoichiometry Molarity Safety Test: SCH3U Review Review: Assignment & text page xiv September 2. Organic Chemistry · · · · Hydrocarbons Functional Groups Reactions of Organic Molecules Polymers Test: Organic Review: Chapters 1, 2 October November TERM 2: UNIT TOPICS 3. Atomic Structure & Molecular Architecture · · · · Atomic Structure Bonding in Solids, Liquids & Gases Molecular Structure Properties of Solids TEST CHAPTER Test: Atomic Structure & Shapes Review: Chapters 3, 4 January TERM 3: UNIT TOPICS 4. Energy & Rates in Chemical Reaction · Thermodynamics · Reaction Rates Test: Thermodynamics Review: Chapters 5, 6 March 5. Introduction to Equilibrium 6. Equilibrium Applications · · · · · Test: Rate & Equilibrium Review: Chapters 6,7 & 9 April Dynamic Equilibrium Le Chatelier’s Principle Equilibrium Problems Solubility Equilibrium Acid-Base Equilibrium TERM 4: UNIT TOPICS 7. Redox & Electrochemistry · Oxidation & Reduction · Electrochemical Cells CHAPTER ISU Partical (May) Review: Chapter 8 CHAPTER Review: Chapters 10, 11 Quest: June