Course Syllabus
advertisement

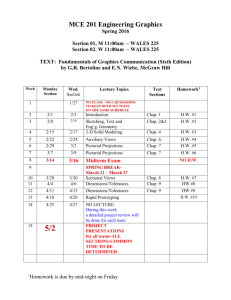
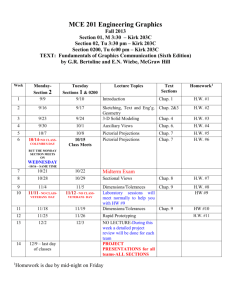
EGR 334 Thermodynamics - 3 credits Instructor: Clark Merkel Office: Science Hall 234 Email: clark.merkel@loras.edu webpage: http://myweb.loras.edu/cm418218 Spring 2012 Phone: office: 563-588-7186 home: 563-513-6896 Meets: MWF from 2:00 to 2:50 p.m. in Science 118 MWF Text: Moran and Shapiro, Fundamentals of Engineering Thermodynamics, 7th ed., Wiley Office Hrs: TBA Course Catalog Description: The laws of thermodynamics. Topics include: properties of substances and phase equilibrium, the first and second laws of thermodynamics, entropy, power cycles and refrigeration cycles. Prerequisites: Chemistry for Engineers (CHE 111 or 114 ), Engineering Dynamics (EGR 232), and Analytic Geometry and Calculus III (MAT 260); or by instructor permission. Objectives: The purpose of this course is to provide an introduction to the principles of thermodynamics. Each student should learn the laws of thermodynamics and how to use these principles when analyzing engineering problems. The class will also cover the analysis techniques of heat cycles, the use of psychometrics, and an introduction to combustion. Topics covered: First law of Thermodynamics; Property evaluation; Second Law of Thermodynamics; Entropy; Heat cycles (Carnot, Otto, Diesel, Brayton); Psychrometrics; Combustion Exams: There will be two exams during the semester, each worth one-half of the exam portion of your grade. You will be allowed one 8 x 10 inch piece of paper and a calculator during the exam. A missed exam will receive a grade of zero unless I have received prior notification of an excused absence. Final Exam: There will be a comprehensive final exam. A missed final exam will receive a grade of zero. Homework: There will be multiple written homework assignments. Homework is due at the beginning of class, and late homework will not be accepted. Your lowest homework score will be thrown out. You may talk to and work with others on the homework, but you must turn in your own solutions. Grading: The weighting that will be used to determine the course grade is shown in the following table: Homework/Quizzes.........……….........30% Exams…………………..………….....40% Final Examination……………………25% Paper…………………………………..5% Academic Dishonesty Dishonesty (cheating, plagiarism, claiming another students work as your own) in class and/or assigned work will result in total loss of credit for the class and/or assigned work. Dishonesty in examinations, which are not final examinations, will result in total loss of credit for the examination. Dishonesty in final examinations will result in a failing grade for the course. All cases of student dishonesty will be reported in writing to the associate vice president for academic affairs by the faculty member. The student may appeal cases of dishonesty to the associate vice president for academic affairs. Attendance: If you miss more than eight classes during the semester, you will receive a failing grade for the course. Learning Disabilities If you have a learning disability, please contact the LD Center to make an appointment with a staff member to determine appropriate and reasonable accommodations. I will be happy to work with you and the center to make the learning and testing procedures used in this course as optimal as possible for you to succeed. Date Reading Assignment Lecture Topics Porblems 1/30 2/1 FRI 2/3 MON 2/6 WED 2/8 FRI 2/10 MON 2/13 WED 2/15 FRI 2/17 MON 2/20 Chap 1 Chap 2.1-2.3 Chap 2.4-2.5 Chap. 2.6 Chap 3.1-3.5 Chap 3.6-3.8 Chap 3.9 Chap 3.10-3.11 Chap 3.12-3.14 Introduction, Review of Units Work and Energy Heat and the Energy Balance Energy Analysis of Cycles Review Chap 2 Problems State Properties Enthalpy and Internal Energy Specific Heats Generalized Compressibility Ideal Gas Model Chap 3.15 Polytropic Processes 2/24 2/27 WED 2/29 FRI 3/2 MON 3/5 WED 3/7 FRI 3/9 MON 3/12 WED 3/14 FRI 3/16 MON 3/19 WED 3/21 FRI 3/23 MON 3/26 Chap 4.1-4.3 Chap 4.4-4.5 Control Volumes CV Conservation of Mass and Energy Exam 1: Chapters 1-3 Fall Free Day*** no class CV Devices and Applications CV and System Analysis Review Chap 4 Problems The 2nd Law of Thermodynamics 2nd Law Applications Carnot Cycle Entropy Entropy in Closed Systems Entropy for Control Volumes Isentropic Processes Chap 1: 19, 37, 51, 59 Chap 2: 6, 20, 24, 32 Chap 2: 49, 53,61, 68 Chap 2: 73, 76, 84, 90 Chap 2: 46, 59, 82,97 Chap 3: 5, 7, 10, 29 Chap 3: 35, 41, 42, 45 Chap 3: 49, 55,68, 78 Chap 3: 92, 93, 96, 99 Chap 3: 102, 107,115, 125 Chap 3: 138, 142,144,147 Chap 4: 1, 6,11, 22 Chap 4: 25,28, 31, 34 3/28 3/30 MON 4/2 WED 4/4 FRI 4/6 MON 4/9 WED 4/11 FRI 4/13 MON 4/16 WED 4/18 FRI 4/20 MON 4/23 WED 4/25 FRI 4/27 MON 4/30 WED 5/2 FRI 5/4 MON 5/7 WED 5/9 FRI 5/11 TBA Chap 8.1-8.2 Chap 8.3-8.4 MON WED WED 2/22 FRI MON WED FRI Chap 4.6-4.9 Chap 4.10-4.12 Chap 5.1-5.3 Chap 5.4-5.9 Chap 5.10-5.11 Chap 6.1 - 6.5 Chap 6.6-6.8 Chap 6.9-6.10 Chap 6.11-6.13 Chap 9.1-9.2 Chap 9.3- 9.4 Chap 9.5-9.6 Chap 9.7-9.8 Chap 10.1-10.4 Chap 10.5-10.7 Chap 12.1 - 12.4 Chap 12.5-12.7 Chap 12.8 Chap 12.9 Chap 13.1 Chap 13.2-12.3 Chap 13.4 Final Exam: Rankine Cycle Rankine Cycle Add ons Review Chap 8 Problems Exam 2 Easter Break******* no class Easter Break******* no class Internal Combustion and Otto Cycle. Diesel and Dual Cycles Gas Turbine and Brayton Cycle Regeneration Refrigeration Systems Heat Pump and Gas Refrigeration Ideal Gas Mixtures Psychrometric Applications Air Conditioning Systems Cooling Towers Introduction to Combustion Combustion and Energy Balance Fuel Cells Review for Final Exam Chap 4: 43, 52, 66, 75 Chap 4: 95, 98, 102, Chap 4: 94, 100, 106 Chap 5: 3, 6, 17, 20 Chap 5: 35,40,43, 56 Chap 5: 64, 79, 81,86 Chap 6: 1,11,21,28 Chap 6: 36, 38, 59, 66 Chap 6: 80, 86, 91, 111 Chap 6: 114, 124, 151,164 Chap 8: 2,7,13,17 Chap 8: 21,29, 49, 60 Paper Chap 9: 1, 3, 11, 14 Chap 9: 17, 20, 24, 38 Chap 9: 42,47, 55,58 Chap 9: 59, 63, 77,80 Chap 10: 2, 4, 9, 21 Chap 10: 34, 39, 43, 48 Chap 12: 4, 10, 22, 28 Chap 12: 46,51, 55, 67 Chap 12: 75, 7882, 92 Chap 12: 103, 106, 107 Chap 13: 1, 6, 10, 21 Chap 13: 46, 51, 54,63 Chap 13: 76, 87 Guidelines for Homework: 1. The procedure and format to be followed in solving problems are illustrated on the attached sheet. Engineering work that is illegible and sloppy is of no value to others who have to use it. Therefore, neatness and systematic solution of problems is an essential trait of good engineering work. 2. System and Control Volume diagrams should be drawn and properly labeled. They represent graphical equations of the problem. Equally important is writing down the standard equation or starting principle from which the problem is developed. Both the diagrams and the developed equations will be reviewed when a problem, quiz, or test is graded. 3. Each credit of academic work is generally considered to mean one hour of class per week and at least two hours of study per hour of class. In other words, you should allow at least a total of 6 hours (possibly more) of outside work each week to be successful in this course. 4. Problem solving format: a) Homework problems should only be presented using one side of each sheet. b) Every sheet should have the individual’s name, course number, and a problem number at the top of the sheet. c) Each problem should be completed on its own sheet. Don’t put more than one problem on a sheet. If a problem requires more than one sheet, successive sheets should also be labeled. d) Every problem should be solved in the following format. Problem Statement: A complete statement of the problem should be given at the start of the problem solution. Known: A summary of the given information is stated Find: A statement of what is wished to be found by the work Starting Principle(s): Identify the starting principle from which the work will be developed. Solution: Diagrams—clear and labeled Equations and calculations Numerical accuracy—how many figures? Answer: Clearly identified with proper units and acceptable numerical accuracy. Comments: State any comments about the answer and whether it seems reasonable and consistent with known information. EME231 9/6/2007 1/4 I. M. Student Problem Statement: An electric motor draws a current of 10 amp with a voltage of 110 V. The output shaft develops a torque of 10.2 N-m and a rotational speed of 1000 rpm. For operation at steady state, determine each in kW. a) the electric power required. 10.2 N-m b) the power developed by the output shaft. 10 A@110V c) the rate of heat transfer. --------------------------------------------------------------1000 rpm Solution: a) Electric power is given by P=IV In this case the input current: I = 10 A the input voltage: V = 110 V. therefore the input power is P = I V = (10A) (110V) = 1100 W b) Rotational Mechanical Power is given by P=Tω where the Torque is: T = 10.2 N-m and the rotational speed: ω = 1000 rpm therefore the output power is P=Tω = (10.2 N-m)( 1000 rev/min)(2π rad/1 rev)(1min/60s) = 1068 N-m/s = 1068 W c) Using the rate form of the energy balance equation: dE Q W dt where for a steady state system: Q ΔE W dE 0 dt Work done by system due to electrical work and mechanical shaft work: W Wout Win Wmech Welec 1068 W 1100 W 32 W Therefore: Q W dE = -32 W dt note that Q is positive for heat flow into the system. So in this case the system has a heat flow rate of 32 W out of the system. Summary: a) Work in: 1100 W b) Work out: 1068 W c) Heat Flow Rate: 32 W from the motor to the environment