Department of Physiology Fall 2003 Seminar Series
advertisement

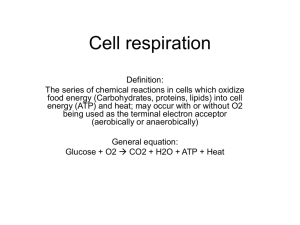
Department of Physiology Fall 2003 Seminar Series Ina Urbatsch, Ph.D. Assistant Professor TTUHSC, Cell Biology & Biochemistry Department Room 5BC 201, Noon –1:00pm, Friday, December 5, 2003 “The Catalytic Mechanism of P-glycoprotein” Multidrug resistance is a major obstacle to cancer chemotherapy because tumors become resistant to the anti-cancer drugs and to a variety of other drugs. Expression of P-glycoprotein (Pgp) in the plasma membrane is one mechanism of multidrug resistance. Pgp is an ATP driven exporter which mediates efflux of a variety of anti-cancer drugs and thereby reduces their intracellular concentration. It belongs to the ABC transporter superfamily. The current working model envisages that Pgp intercepts drugs from the inner leaflet of the plasma membrane before they reach the cytoplasm, and pumps them out using ATP hydrolysis as an energy source. We have studied kinetics of inhibition of Pgp ATPase using the transition state analog vanadate. Incubation with vanadate in the presence of MgATP or MgADP produces stable inhibition of ATPase activity and trapping of MgADP in one of the two catalytic sites. Verapamil and anti-cancer drugs strongly magnified the effects. Catalytic site mutants prevented trapping whereas interdomain signal communication mutants reduced trapping in correlation with their effects on ATPase activity. In explanation of our results, we propose that a “closed conformation” involving dimerization and interdigitation of the two ATP sites is necessary to allow inhibition by ADP-vanadate. The results suggest that such a dimerization occurs naturally during ATP hydrolysis. It is proposed that in order for the catalytic transition state to form, the two ATP sites dimerize to form an integrated single entity containing two bound ATP with just one of the two ATP being hydrolyzed per dimerization event. Currently, our laboratory is trying to express other ABC transporters involved in multidrug resistance and genetic disease in the yeast Pichia pastoris. One of them is the Breast Cancer Resistance Protein (BCRP) which is a “half” transporter suggested to function as a homo-dimer. We are trying to purify these proteins in large scale to provide material for functional studies and for structure determination by x-ray crystallography. Our effort will facilitate understanding of the molecular basis of drug transport and will provide biochemical and structural foundation for rational design of better and more potent drugs to circumvent multidrug resistance of human cancers.. Recent Publications: 1. Urbatsch, I.L., Al-Shawi, M.K., and Senior, A.E. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry 1994; 33: 7069-7076. 2. Al-Shawi, M.K., Urbatsch, I.L., and Senior, A.E. Covalent inhibitors of P-glycoprotein ATPase activity. J. Biol. Chem. 1994; 269: 8986-8992. 3. Urbatsch, I.L., and Senior, A.E. Effects of lipids on ATPase activity of purified Chinese hamster P-glycoprotein. Arch. Biochem. Biophys. 1994; 316: 135-140. Persons with disabilities who may need auxiliary aids or services are requested to contact Cindy Hutson (806-743-2520) at least 24 hours prior to this seminar so that appropriate arrangements can be made. 4. Senior, A.E., Al-Shawi, M.K., and Urbatsch, I.L. ATP hydrolysis by multidrug-resistance protein from Chinese hamster ovary cells. J. Bioenerg. Biomembr. 1995, 27; 31-16. 5. Urbatsch, I.L., Sankaran, B., Weber, A., and Senior, A.E. P-plycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J. Biol. Chem. 1995, 270: 19383-19390. 6. Urbatsch, I.L., Sankaran, B., Baghat, S., and Senior, A.E. Both P-glycoprotein nucleotide binding sites are catalytically active. J. Biol. Chem. 1995; 270: 26956-26961. Persons with disabilities who may need auxiliary aids or services are requested to contact Cindy Hutson (806-743-2520) at least 24 hours prior to this seminar so that appropriate arrangements can be made.